Abstract
Purpose
The aim of this study is to assess the efficacy and complications of trabeculectomy with a biodegradable implant (ologen implant) vstrabeculectomy using mitomycin C (MMC) in patients with medically uncontrolled open-angle glaucoma in a prospective randomised clinical trial.
Methods
In the MMC group (10 patients), trabeculectomy was performed according to standard protocols. In the ologen group (10 patients) after standard trabeculectomy the implant was positioned on top of the scleral flap and no MMC was applied. Follow-up was continued for 12 months after surgery and included testing of intraocular pressure (IOP), visual acuity, visual field, ultrasound biomicroscopy, and filtering bleb score.
Results
The mean preoperative IOP was 24.8±8.9 mm Hg for all patients enrolled. At 1 year after surgery, the mean IOP was 15.6±2.4 mm Hg in the ologen group (P<0.01, 43% reduction) and 11.5±4.1 mm Hg in the MMC group (P<0.01, 50% reduction). No anti-glaucomatous medication was necessary in the MMC group in the first year of follow-up, whereas five patients in the ologen group required topical treatment. The absolute success rate was 100% in the MMC group and 50% in the ologen group (P=0.01). After 1 year, filtering blebs developed significantly more avascular areas in the MMC group (score=1.4) than in the ologen group (score=2.8; P<0.01).
Conclusion
The complete success rate using trabeculectomy with the ologen implant is lower than that achieved by trabeculectomy with MMC. However, the bleb morphology caused more problems in the MMC group (avascularity score).
Similar content being viewed by others
Introduction
Long-term intraocular pressure (IOP) control following trabeculectomy may be limited by filtration failure, because of scarring at the level of the conjunctiva–Tenon's–episcleral interface, the scleral flap, its overlying episclera, or the internal ostium.1 Adjunctive anti-fibrotic agents such as 5-fluorourail or mitomycin C (MMC) have a potent inhibitory effect on postoperative scarring and a positive effect on surgical success rates.2, 3 Although the risk profile of additional use of MMC in trabeculectomy appears low, some side effects such as cataract formation, avascular filtering blebs, thinning of the conjunctiva, subsequent blebitis, and endophthalmitis have been described.3, 4, 5, 6
Recently, tissue-engineered biodegradable implants have been created as an alternative augmentation in trabeculectomy. Tissue engineering involves the combination of a polymer scaffold with a population of stem, progenitor, or precursor cells. Tissue growth is modelled to favour the development of a particular structure and, if the polymer scaffold used is biodegradable, can result in the formation of structures, which are remarkably similar to normal tissue.7 Various different implants are in production. In animal models a porous collagen–glycosaminoglycan matrix (ologen) has been tested. This implant should prevent the collapse of the subconjunctival space. In animal studies, randomised collagen deposition and microcyst formation after penetrating anti-glaucomatous surgery have been shown in the ologen group8, 9 in contrast to negative controls. Moreover, the biodegradable implant decreased early postoperative scarring.8 A prospective pilot study revealed no significant difference in the postoperative IOP after trabeculectomy in the control group and trabeculectomy with ologen in the study group.10
In a prospective, randomised study with two interventional groups, we investigated the ologen implant as an alternative for modulation of wound healing in anti-glaucomatous surgery. As a control, we selected the trabeculectomy with intraoperative application of MMC, which we currently used in our department before the new material became available. Our study focused on postoperative IOP, anti-glaucomatous medication, and filtering bleb morphology.
Materials and methods
Patients and preoperative examination
We initiated a randomised, prospective trial with two study groups undergoing penetrating anti-glaucomatous surgery (trabeculectomy). Group A comprised patients undergoing standard trabeculectomy with application of 0.2 mg/ml MMC for 3 min, whereas group B comprised patients undergoing standard trabeculectomy and the implantation of one ologen-implant (version 1). The plan was to include a consecutive series of 40 patients (20 to each group). Randomisation was performed by an individual not involved in the study according to the Consort Guidelines description. After the first 20 patients, an interim analysis was prearranged. Patients were included in the study if they had primary or secondary open-angle glaucoma with uncontrolled IOP while receiving maximal tolerable anti-glaucomatous therapy. Patients with angle-closure glaucoma, post-traumatic, uveitic, neovascular, or dysgenetic glaucoma were not considered for this study. Patients with an allergy to collagen, preliminary conjunctival damage (trauma, vitreo–retinal surgery, previous glaucoma surgery, and other) or those <18 years of age were excluded from the study. A supplied collagen allergy test was applied before surgery. Possible alternatives, beneficial effects, and potential complications of the surgical procedures were explained in detail to all patients. Written informed consent for surgery and for inclusion in a prospective randomised trial was obtained from all participants. Before surgical intervention all patients underwent a baseline examination, which included measurement of best-corrected visual acuity (ETDRS charts, Lighthouse, Long Island, NY, USA), visual field examination (30-2, Humphrey field analyser, Humphrey Instruments, Munich, Germany), biomicroscopy, gonioscopy, and Goldmann applanation tonometry.
The trial conforms to the standards set by the Declaration of Helsinki and was approved by the local ethics committee.
Ologen implant (version 1)
The ologen implant (version 1) (Aeon Astron Europe BV, Leiden, The Netherlands) is a porous implant comprising >90% lyophilised porcine collagen and <10% lyophilised glycosaminoglycan with a pore size of 10–300 μm. In our study, we used a cylindrical (7 mm diameter) implant of 4 mm in height.
Surgical technique and follow-up
The surgical procedure was performed under either general or local anaesthesia, according to the patient's preference. Operations were performed as follows: The procedure commenced with the creation of a fornix-based conjunctival flap in an upper quadrant. Careful monopolar cauterisation was performed on the surface of the sclera. Only in study group A (trabeculectomy with MMC) was a sponge with MMC (0.2 mg/ml) positioned on the scleral surface for 3 min. Afterwards, MMC was rinsed out with balanced salt solution. In both groups a limbus-based triangular flap of two-thirds of the scleral thickness was dissected. The size of the flap was standardised to a size of 5 mm basis at the limbus. The sclerotomy of 1 × 1 mm was performed, followed by a peripheral iridectomy. The scleral flap was then repositioned and fixed with one loose-fitting 10-0 nylon suture in the ologen group and a tight suture in the MMC group, according to the manufacturer's recommendations and protocols in previous publications.8 Only in study group B (trabeculectomy with ologen implant) a cylindrical ologen implant (7 mm diameter) was positioned on top of the scleral flap, to exert pressure on the scleral flap to avoid postoperative hypotonia. Finally, the conjunctiva was closed with a 9-0 polyglactin suture (Vicryl, Ethicon Limited, Edinburgh, UK). The standard postoperative regimen consisted of topical ofloxacin three times a day and dexamethasone five times a day in a preservative-free preparation.
Postoperative examinations were performed on a daily basis during hospitalisation. Follow-up visits were arranged at 1 and 2 weeks and 1, 2, 3, 6, and 12 months after surgery. At each follow-up visit, all the aforementioned examinations except visual field testing and gonioscopy were repeated. Visual field examination was repeated 6 and 12 months after surgery. Side effects and complications were recorded during postoperative visits. Complications were defined as follows: encapsulated filtering bleb (Tenon's cyst), shallow anterior chamber, hyphema, ablation of the choroidals (eg, choroidal effusion), persistent leakage, hypotony (IOP <6 mm Hg), macular oedema, corneal complications, allergy, suprachoroidal haemorrhage, and blebitis/endophthalmitis.
A bleb classification score estimating the avascularity, corkscrew vessels, and microcysts of the bleb was noted at every time point during follow-up by a member of the study team, as in the Würzburg Bleb Classification.11 Three parameters (avascularity, corkscrew vessels, and microcysts) were scored from 0 to 3. The scoring was performed by a member of the study team at the time of follow-up assessment. In case of avascularity and corkscrew vessels, scoring was performed as follows: 0, entire bleb; 1, in two-thirds of the bleb; 2, in one-third of the bleb; 3, none. In case of microcysts, scoring was carried out as follows: 0, none; 1, over the scleral flap; 2, lateral and medial to the scleral flap; 3, entire bleb.
Statistics
Complete success was defined as an IOP of ⩽18 mm Hg and a relative decrease of ⩾20%, compared with the preoperative IOP, without any additional glaucoma surgery or medication. Qualified success was defined as an IOP of ⩽18 mm Hg and an additional reduction of ⩾20% in IOP compared with the preoperative IOP, without any additional glaucoma surgery, but with one topical medication allowed. Particular attention was paid to postoperative complications.
Statistical analysis was performed using Prism software (version 2, GraphPad Software Inc., La Jolla, CA, USA). Surgical success was compared using the log-rank test and survival curves (Kaplan–Meier curves). Differences between preoperative and postoperative IOP and medication were compared by the non-parametric t-test (Mann–Whitney test, two tailed). The bleb classification data were considered as non-parametric; statistical analysis was performed using the Mann–Whitney test. Comparison of complications in the interventional groups was done with the χ2-test. P-values of <0.05 were considered statistically significant.
Results
Twenty (10/10) patients, 12 women and 8 men, began the study, and it was completed by 19 patients. One patient from the MMC group died 8 months after surgery. The mean age was 62.8±9.5 years. All patients were of Caucasian ancestry. Fourteen patients had primary open-angle glaucoma and six patients had secondary open-angle glaucoma, each of the latter having pseudoexfoliation glaucoma. The mean preoperative IOP was 24.8±8.9 mm Hg for all patients enrolled. No difference in mean preoperative IOP was observed between the two groups (P=0.24), nor was any difference in preoperative medication noted (P=0.75).
IOP and medication
At 1 day after surgery the mean postoperative IOP was 10.1±5.6 mm Hg for the ologen group (P<0.001 compared with preoperative IOP) and 10.8±4.7 mm Hg for the MMC group (P<0.001), respectively. During the first 2 weeks after surgery, no significant difference in IOP and medication score was detectable between the two groups (see Table 1). At 4 weeks after surgery, the mean IOP in the ologen group rose to 14.3±4.7 mm Hg. As the pressure in the MMC group remained at a low level (9.4±3.2 mm Hg), a significant difference between the two groups was detected at this point in follow-up. The pressure in both groups remained still stable over 12 months of follow-up after surgery (see Table 1). The pressure level in the MMC group was ∼4 mm Hg lower than that in the ologen group. A significant difference in IOP was determined from 1 month to 1 year after surgery.
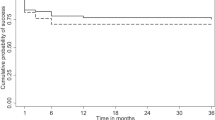
In 1 year of follow-up, no patient in the MMC group required anti-glaucomatous medication to achieve the target IOP. Instead, in the ologen group, five patients needed a topical therapy (mean number of medication 0.8±1.1; P=0.07). In the MMC group, complete success was achieved in 100% of cases, whereas it was only 50% in the ologen group. In the Kaplan–Meier chart for complete success (Figure 1), the course of the curves was dispersed. After interim analysis, the study was aborted because of the significantly lower IOP and significantly higher complete success rate in the MMC group after 1 year (P=0.01).
Complications and side effects
During postoperative follow-up visits, we could not detect any possible ologen specific side effects such as allergy or translocation of the implant. Table 2 gives an overview of the recorded side effects. Using the χ2-test, we could not detect any significant difference between the two groups. Interestingly, hypotony was very common in the early postoperative stage (<7 days postoperatively) in the ologen group (6 of 10 cases). However, a shallow anterior chamber occurred in only two cases. No significant difference between the two groups was detectable. At 1 week after surgery, no hypotony was observed in the ologen group, whereas hypotony was detectable in 3 of 10 cases in the MMC group. In two cases in the ologen group, Tenon's cysts built up after 4 weeks postoperatively and needling was necessary. In the MMC group revision, surgery was necessary because of a prominent bleb cyst and a subsequent late leakage in one case.
In all the 19 cases observed, the visual acuity remained stable over a period of 1 year. Stability in the visual field was also observed over 1 year in all cases.
Bleb morphology
Filtering blebs formed in both the interventional groups. Nevertheless, we detected several differences in the morphology of the blebs.
In the early postoperative stage (up to 4 weeks postoperatively), filtering blebs in the ologen group were more prominent because of the subconjunctival implant than in the MMC group. At 1 month after surgery, the ologen implant was no longer detectable on ultrasound biomicroscopy. Filtering blebs in the MMC group showed a thinning of the conjunctiva after 6 and 12 months and often pronounced avascular zones (Figure 2). A single filtering bleb of the MMC group showed a prominent avascular zone with leakage 3 months after surgery, requiring revision. In the ologen group the filtering blebs did not show any avascular areas (Figure 3). The described bleb classification score was used for objectivation (Table 3). No difference was detectable for corkscrew vessels at any time after surgery. Otherwise, filtering blebs of the MMC group showed significantly more microcysts (score=1.8±0.63) after 12 months than the blebs of the ologen group (score=1.00±0.82; P=0.02). Furthermore, MMC blebs showed significantly more avascular areas than blebs of the ologen group. A significant difference was revealed from 1 to 12 months after surgery. Altogether, filtering blebs of the MMC group showed a more prominent and avascular filtration and more microcysts, whereas blebs in the ologen group were more flat, with diffuse and expanded filtration (Figures 2 and 3).
Discussion
Penetrating anti-glaucomatous surgical procedures allow a powerful reduction of IOP. The pressure-reducing effect of penetrating surgery is probably still higher than that of non-penetrating strategies, particularly in the long run.12, 13, 14 However, postoperative scarring is a major problem, compromising postoperative surgical success in both groups.
Trabeculectomy as the standard procedure in penetrating anti-glaucomatous surgery was introduced by Cairns in 1968.15 The method was developed further over subsequent decades to address various problems. In 1990, MMC was applied as an anti-metabolite during trabeculectomy.16 Various studies demonstrated significant enhancement of success rates and postoperative IOP through intraoperative use of MMC.3 This was accompanied by an increase in adverse effects such as cataract formation, avascular filtering blebs, thinning of the conjunctiva, subsequent blebitis, and endophthalmitis.3, 4, 5, 6 The current focus is on the development of less toxic agents and implants to inhibit cicatrisation without adverse effects. One approach is the development of biodegradable implants to serve as a placeholder and prevent conjunctiva–sclera adhesion.
A few different biodegradable implants are due to be tested in animal models. With a poly(L-lactide-co-epsilon-caprolactone) film, designed to work as an adhesion barrier in filtration surgery, a significantly lower postoperative IOP was found in relation to control eyes and no significant difference to outcome in MMC-treated eyes was detected.17 A solid hyaluronic acid–carboxymethyl cellulose film significantly inhibited subconjunctival scar formation and prevented adhesions of conjunctiva and sclera.18 The use of seprafilm (sodium hyaluronate and carboxymethylcellulose) reduced postoperative conjunctiva–sclera adhesion.19 A porous collagen–glycosaminoglycan matrix (ologen implant) was tested in animal models. This implant was designed to prevent collapse of the subconjunctival space, for example, the conjunctiva–sclera adhesion. It led to a randomised collagen deposition and microcyst formation after penetrating anti-glaucomatous surgery in contrast to the negative control and decreased early postoperative scarring.8, 9 Moreover, the ologen implant will also be adjuvant in repairing postoperative bleb leaks.9 In human subjects, the ologen implant was tested for augmentation in deep sclerectomy. This study revealed that deep sclerectomy with ologen implantation is an effective and well-tolerated method for reduction of IOP.20 A further pilot study revealed non-significant differences in postoperative IOP after trabeculectomy with ologen and sole trabeculectomy.10 In summary of the previous studies, the use of the ologen implant promises comparable IOP reduction after trabeculectomy and a lower risk profile in comparison with the use of anti-metabolites, for example, MMC and 5-fluorouracil, although the use of ologen implant does not seem to offer a significant advantage compared with trabeculectomy alone in a pilot study.10
Our study reveals that trabeculectomy with implantation of an ologen implant is a safe method for penetrating anti-glaucomatous surgery. We did not detect any ologen-specific side effects, such as translocation of the implant or erosion of the conjunctiva. No allergy was detected and corkscrew vessel scores were comparable in the two interventional groups. With our surgical protocol, using a single loose-fitting suture of the scleral flap, early hypotony was common in the ologen group.
Although IOP in the early postoperative course was comparable in the two groups, after 1 month a significant difference (P=0.01) in IOP emerged. This difference persisted until 12 months after surgery (Table 1). With regard to the need for topical anti-glaucomatous drugs in five cases in the ologen group (50% complete success) and the complete success rate of 100% in the MMC group, MMC is clearly more effective for augmentation in trabeculectomy. Nevertheless, filtering bleb morphology as described here caused more problems in the MMC group, and only in this group was persistent leakage, requiring surgical intervention observed.
Although previous studies have promised favourable outcome of ologen use in glaucoma surgery, we have to face a significantly better absolute success rate of traditional filtering surgery with MMC use compared with filtering surgery combined with the ologen implant. Obviously, the use of MMC (0.2 mg/ml) has a stronger effect on the vascularity and transparency of the filtering bleb than the subconjunctival positioning of an ologen implant.
Further modifications of the ologen implant in terms of size, surgical technique, and also molecular structure will be needed to improve the functional outcome. An interesting new development is the ologen implant made of atelocollagen (version 2), which may reduce inflammatory reaction within the surrounding tissue. Atelocollagen obtained by pepsin treatment is low in immunogenicity because it is free from telopeptides.21 Further investigations are warranted to establish the future role of the ologen implant in glaucoma surgery.

References
Azuara-Blanco A, Katz LJ . Dysfunctional filtering blebs. Surv Ophthalmol 1998; 43: 93–126.
Martini E, Laffi GL, Sprovieri C, Scorolli L . Low-dosage mitomycin C as an adjunct to trabeculectomy. A prospective controlled study. Eur J Ophthalmol 1997; 7: 40–48.
Wilkins M, Indar A, Wormald R . Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev 2005 Issue 4. Art. no.: CD002897..
Mac I, Soltau JB . Glaucoma-filtering bleb infections. Curr Opin Ophthalmol 2003; 14: 91–94.
Reibaldi A, Uva MG, Longo A . Nine-year follow-up of trabeculectomy with or without low-dosage mitomycin-c in primary open-angle-glaucoma. Br J Ophthalmol 2008; 92: 1666–1670.
Beckers HJ, Kinders KC, Webers CA . Five-year results of trabeculectomy with mitomycin C. Graefes Arch Clin Exp Ophthalmol 2003; 241: 106–110.
Young MJ, Borras T, Walter M, Ritch R . Potential applications of tissue engineering in glaucoma. Arch Ophthalmol 2005; 123: 1725–1731.
Chen HS, Ritch R, Krupin T, Hsu WC . Control of filtering bleb structure through tissue bioengineering: An animal model. Invest Ohthalmol Vis Sci 2006; 47: 5310–5314.
Hsu WC, Ritch R, Krupin T, Chen HS . Tissue bioengineering for surgical bleb defects: an animal study. Graefes Arch Clin Exp Ophthalmol 2008; 246: 709–717.
Papaconstantinou D, Georgalas I, Karmiris E, Diagourtas A, Koutsandrea C, Ladas I et al. Trabeculectomy with OloGen versus trabeculectomy for the treatment of glaucoma: a pilot study. Acta Ophthalmol 2010; 88: 80–85.
Klink T, Schrey S, Elsesser U, Klink J, Schlunck G, Grehn F . Interobserver variability of the Würzburg bleb classifiaction score. Ophthalmologica 2008; 222: 408–413.
Lüke C, Dietlein TS, Jacobi PC, Konen W, Krieglstein GK . A prospective randomized trial of viscocanalostomy versus trabeculectomy in open-angle glaucoma: a 1-year follow-up study. J Glaucoma 2002; 11: 294–299.
Cillino S, Di Pace F, Casuccio A, Calvaruso L, Morreale D, Vadalà M et al. Deep sclerectomy versus punch trabeculectomy with or without phacoemulsification: a randomized trial. J Glaucoma 2004; 13: 500–506.
Chiselita D . Non-penetrating deep sclerectomy versus trabeculectomy in primary-open-angle glaucoma surgery. Eye 2001; 15: 197–201.
Cairns JE . Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 1968; 66: 673–679.
Chen CW, Huang HT, Bair JS, Lee CC . Trabeculectomy with simultaneous topical application of mitomycin-c in refractory glaucoma. J Ocul Pharmacol 1990; 6: 175–182.
Okuda T, Higashide T, Fukuhira Y, Sumi Y, Shimomura M, Sugiyama K . A thin honeycomb-patterned film as an adhesion barrier in an animal model of glaucoma filtration surgery. J Glaucoma 2009; 18: 220–226.
Takeuchi K, Nakazawa M, Yamazaki H, Miyagawa Y, Ito T, Ishikawa F et al. Solid hyaluronic acid film and the prevention of postoperative fibrous scar formation in experimental animal eyes. Arch Ophthalmol 2009; 127: 460–464.
Tsurumaru N, Arai M, Teruya K, Sueda J, Yamakawa R . Seprafilm as a new antifibrotic agent following trabeculectomy in rabbit eyes. Jpn J Ophthalmol 2009; 53: 164–170.
Aptel F, Dumas S, Denis P . Ultrasound biomicroscopy and optical coherence tomography imaging of filtering blebs after deep clerectomy with new collagen implant. Eur J Ophthalmol 2009; 19: 223–230.
Stenzel KH, Miyata T, Rubin AL . Collagen as a biomaterial. Annu Rev Biophys Bioeng 1974; 3: 231–253.
Acknowledgements
PJ Dahlhausen & Co. GmbH (Emil-Hoffmann-Str. 53, 50996 Cologne, Germany) supported the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The sponsor had no role in the design or conduct of this research.
Rights and permissions
About this article
Cite this article
Rosentreter, A., Schild, A., Jordan, J. et al. A prospective randomised trial of trabeculectomy using mitomycin C vs an ologen implant in open angle glaucoma. Eye 24, 1449–1457 (2010). https://doi.org/10.1038/eye.2010.106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.106
Keywords
This article is cited by
-
Revision des PRESERFLO® MicroShunts mit Ologen und Mitomycin C
Die Ophthalmologie (2023)
-
Comparison of surgical outcomes with and without Ologen collagen matrix augmentation during XEN gel stent implantation
BMC Ophthalmology (2022)
-
Subconjunctival implantation of ologen Collagen Matrix to treat ocular hypotony after filtration glaucoma surgery
Eye (2017)
-
Biodegradable collagen matrix implant versus mitomycin-C in trabeculectomy: five-year follow-up
BMC Ophthalmology (2016)
-
Collagen matrix vs mitomycin-C in trabeculectomy and combined phacoemulsification and trabeculectomy: a randomized controlled trial
BMC Ophthalmology (2016)