A sperm cell (right) swims towards a human egg (artificially coloured).Credit: AJ Photo/Science Photo Library
The day when human sperm and eggs can be grown in the laboratory has inched a step closer, with the discovery of a way to recreate a crucial developmental step in a dish1.
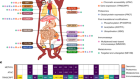
The advance, described on 20 May in Nature, addresses a major hurdle: how to ensure that the chemical tags on the DNA and associated proteins in artificially produced sperm and eggs are placed properly. These tags are part of a cell’s ‘epigenome’ and can influence whether genes are turned on or off. The epigenome changes over a person’s lifetime; during the development of the cells that will eventually give rise to sperm or eggs, these marks must be wiped clean and then reset to their original state.
“Epigenetic reprogramming is key to making the next generation,” says Mitinori Saitou, a stem-cell biologist at Kyoto University in Japan, and a co-author of the paper. He and his team worked out how to activate this reprogramming — something that had been one of the biggest challenges in generating human sperm and eggs in the laboratory, he says.
But Saitou is quick to note that there are further steps left to conquer, and that the epigenetic reprogramming his lab has achieved is not perfect.
“There is still much work to be done and considerable time required to address these challenges,” agrees Fan Guo, a reproductive epigeneticist at the Chinese Academy of Sciences Institute of Zoology in Beijing.
Eggs in a dish
Growing human sperm and eggs in the laboratory would offer hope to some couples struggling with infertility. It would also provide a way to edit disease-causing DNA sequences in sperm and eggs, sidestepping some of the technical complications of making such edits in embryos. And understanding how eggs and sperm develop can give researchers insight into some causes of infertility.
Making mice with two dads: this biologist rewrote the rules on sexual reproduction
But in addition to its technical difficulty, growing eggs and sperm in a dish — called in vitro gametogenesis — would carry weighty social and ethical questions. Genetic modification to prevent diseases, for example, could lead to genetic enhancement to boost traits associated with intelligence or athleticism.
Epigenetic reprogramming is key to the formation of reproductive cells — without it, the primordial cells that would eventually give rise to sperm and eggs stop developing. Furthermore, the epigenome affects gene activity, helping cells with identical DNA sequences to take on unique identities. The epigenome helps to differentiate a brain cell, for example, from a liver cell.
Researchers know how to grow mouse eggs and sperm using stem-cell-like cells generated from skin. But the protocols used don’t work in human cells: “There is a big gap between mice and humans,” says Saitou.
Pressing reset on the epigenome
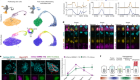
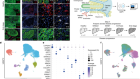
So Saitou and his colleagues began an arduous search for a way to control epigenetic reprogramming in human cells. They found that a protein called BMP2 was essential for this step and that adding it to their cultures promoted epigenetic reprogramming. The cells grown in this culture were able to progress a step further in their development than were cells in cultures without added BMP2.
After epigenetic reprogramming, the cells’ development halted again. Even so, each step towards in vitro gametogenesis holds “immense significance”, says Guo. Saitou and his colleagues are now hunting for ways to nudge the cells further along the path to becoming sperm and eggs.
Embryos with DNA from three people develop normally in first safety study
The researchers carefully analysed epigenetic marks in their laboratory-grown cells and found that although many of these imprints had been wiped away, a few remained. This means that the reprogramming might be incomplete — which could have serious consequences if such cells were used for reproduction. “If imprinting on even one gene is aberrant, that could lead to disease,” says Saitou.
Such caveats are important to bear in mind, he says: the field of in vitro gametogenesis is advancing rapidly, and these results, along with other developments in the past few years, could fuel speculation and false claims that a solution is just around the corner. “I think in maybe five years or so, things will get more settled,” he says. “And then only the good science will remain.”

 What’s next for lab-grown human embryos?
What’s next for lab-grown human embryos?
 The mice with two dads: scientists create eggs from male cells
The mice with two dads: scientists create eggs from male cells
 Mouse embryos grown without eggs or sperm: why, and what’s next?
Mouse embryos grown without eggs or sperm: why, and what’s next?