Antibiotic-resistant bacteria, especially those that are resistant to multiple drugs, pose a major threat to health globally; tackling them is a crucial research priority of the World Health Organization. I am a protein chemist and molecular biologist investigating why bacteria often refuse to die when attacked by antibiotics.
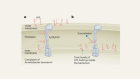
Bacteria can evade antibiotics in clever ways. One involves ejecting them through efflux pumps embedded in the bacterium’s inner membrane. These are like tiny vacuum cleaners that constantly flush out substances that are toxic to bacteria.
I’ve been fascinated by efflux pumps ever since my undergraduate studies in Mexico more than 16 years ago. I’m particularly interested in how they can bind to and extrude dozens of chemically unrelated compounds quickly, efficiently and simultaneously. I’m working on the hypothesis that they do this using a unique group of proteins that lack stable structures — known as intrinsically disordered proteins.
Here at the Pasteur Institute in Lille, France, I’m using advanced screening technology to test the ability of different molecules to inhibit the actions of efflux pumps. I’m helped by an automated circuit, with a range of experimental equipment, and a robot that can load up to 200 assay plates into a carousel. I can program in what I want and go for a coffee while the robot gets to work.
I find it amazing that the proteins in all life forms consist of different combinations of the same 20 amino acids. I can’t really describe why, but solving problems about proteins makes me so happy.
With colleagues, I’ve also been working to develop new efflux pump inhibitors (C. Plé et al. Nature Commun. 13, 115; 2022). My hope is that these can be used both to advance our understanding of how efflux pumps work and, with existing antibiotics, to counter antibiotic and multidrug resistance.


 The staggering death toll of drug-resistant bacteria
The staggering death toll of drug-resistant bacteria
 The antibiotic paradox: why companies can’t afford to create life-saving drugs
The antibiotic paradox: why companies can’t afford to create life-saving drugs
 Nature Outlook: Antimicrobial resistanc
Nature Outlook: Antimicrobial resistanc