The antibiotic drug candidate zosurabalpin killed multiple resistant strains of Acinetobacter baumannii (pictured) in culture and one fully resistant strain in mice.Credit: George Mattei/SPL
The bacterium Acinetobacter baumannii is a portrait of resilience. The microorganism causes a range of infections, and its ability to survive desiccation means it can persist for weeks on hospital air vents, computer keyboards and human skin. Its metabolic and genetic flexibility have allowed it to become resistant to the few antibiotics that can make it through its two protective cell membranes. Antibiotic-resistant microbes kill more than one million people each year. The global threat posed by A. baumannii has put the microbe high on the list of priority pathogens drawn up by the World Health Organization (WHO).
Two studies published on 3 January in Nature report a new class of drug candidates for tackling A. baumannii infections (C. Zampaloni et al. Nature https://doi.org/10.1038/s41586-023-06873-0 (2023); K. P. Pahil et al. Nature https://doi.org/10.1038/s41586-023-06799-7; 2023). One of these compounds has already made it into clinical trials, but it is still a long way from being approved for clinical use.
A new type of antibiotic targets a drug-resistant bacterium
The obstacles for developing such compounds are not just scientific: the economic incentives are insufficient for many companies to take the risk. As the threat of resistance grows, the international community must do more to shepherd promising drugs from bench to bedside.
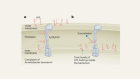
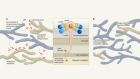
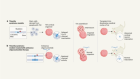
The new compounds block the bacterium’s ability to shuttle key building blocks to where they are needed, and do so by binding to a novel site in the bacterium — there are other compounds that target this pathway, but A. baumannii is resistant to them. One of the molecules, called zosurabalpin, killed multiple resistant strains of A. baumannii in culture, and, in mice, a strain resistant to all available antimicrobials. The first clinical-trial results for the compound are expected this year.
It is rare to get antibiotics with a new mode of action into the clinic — only one in 30 candidates makes it as far as testing in people. Even when a new drug does get through clinical trials and is approved for use, it is often held in reserve for worst-case scenarios, for fear that widespread use would hasten the day when microbes develop resistance to it.
Clearing the initial phase of clinical trials will be only the beginning. Further studies will then be needed to assess the risk of resistance to zosurabalpin emerging in clinical settings. Then, if the drug is approved, someone will need to pay for it. The commercial market is dismal by design. A new antibiotic often costs more than US$1 billion to develop, but the reluctance to use it widely means that it is likely to earn less than $100 million a year once on the market. The WHO and others have warned governments about the scale of the expense, coupled with the growing threat of antimicrobial resistance. Some governments have responded with incentives to encourage industry to take up the challenge. But there has been much more talk than action.
Read the paper: A novel antibiotic class targeting the lipopolysaccharide transporter
The solution, health economists say, is a mix of incentives for new antimicrobial drug development. ‘Push’ strategies are designed to reduce the costs and can include more government funding for early-stage research. ‘Pull’ approaches reward companies for developing successful antibiotics — for example, governments might guarantee a minimum level of purchase, similar to the advanced purchases made of vaccines during the COVID-19 pandemic. Governments have tended to lean more towards push strategies. Economists say they need to do more pulling. But they must also heed lessons learnt from COVID-19 vaccines, such as ensuring that pricing and contracts are transparent, which did not happen during the pandemic.
The United Kingdom has been a leader in this regard. In 2019, it launched a ‘subscription’ programme through which companies are paid a fixed annual fee that is based on a drug’s value to the health-care system, rather than on the number of doses sold. Other countries are considering similar plans. In the United States, a bipartisan team of lawmakers is backing the Pioneering Antimicrobial Subscriptions to End Upsurging Resistance (PASTEUR) Act, which would create a similar programme. But the act has struggled in the US Congress since it was first introduced in 2020. The European Union has also been unable to pass relevant legislation. It is time for governments to move from consideration to action.
Some hesitation is understandable: the subscription model would put further pressure on health-care budgets that are already feeling the strain of drug prices fuelled by high levels of inflation. But there’s another way to look at such payments: as insurance against future health-care crises. If governments can make the market for these drugs viable, that will also encourage companies to invest.
In September, the United Nations General Assembly will host a high-level meeting to discuss antimicrobial resistance — the first such meeting since 2016. This will highlight the problem and offer an opportunity to extract real commitments from member states. The last meeting was transformational in one respect: more than 150 countries agreed to draw up action plans to address antimicrobial resistance.
But an action plan is not the same as taking action — many plans are neither fully implemented nor fully funded. That must change. The discovery of compounds that show promising preclinical activity is precisely what the doctor ordered. But without a strategic funding plan, this prescription could be sitting on the shelf for years. All the while, A. baumannii and its ilk will continue to pose an urgent health-care threat with limited treatment options.

 A new type of antibiotic targets a drug-resistant bacterium
A new type of antibiotic targets a drug-resistant bacterium
 Read the paper: A novel antibiotic class targeting the lipopolysaccharide transporter
Read the paper: A novel antibiotic class targeting the lipopolysaccharide transporter
 Read the paper: A new antibiotic traps lipopolysaccharide in its intermembrane transporter
Read the paper: A new antibiotic traps lipopolysaccharide in its intermembrane transporter
 The staggering death toll of drug-resistant bacteria
The staggering death toll of drug-resistant bacteria
 Why the pandemic treaty risks becoming COVID-19 groundhog day
Why the pandemic treaty risks becoming COVID-19 groundhog day