- NEWS AND VIEWS
An unexpected trigger for calorie burning in brown fat
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Nature 560, 38-39 (2018)
doi: https://doi.org/10.1038/d41586-018-05619-7
References
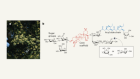
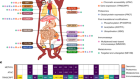
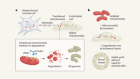
Mills, E. L. et al. Nature 560, 102–106 (2018).
Cannon, B. & Nedergaard, J. Physiol. Rev. 84, 277–359 (2004).
Rosen, E. D. & Spiegelman, B. M. Cell 156, 20–44 (2014).
Nedergaard, J., Ricquier, D. & Kozak, L. P. EMBO Rep. 6, 917–921 (2005).
Harms, M. & Seale, P. Nature Med. 19, 1252–1263 (2013).
Carey, A. L. et al. Diabetologia 56, 147–155 (2013).
Chouchani, E. T., Kazak, L. & Spiegelman, B. M. J. Biol. Chem. 292, 16810–16816 (2017).
Enerbäck, S. Cell Metab. 11, 248–252 (2010).
Hui, S. et al. Nature 551, 115–118 (2017).

 Read the paper: Accumulation of succinate controls activation of adipose tissue thermogenesis
Read the paper: Accumulation of succinate controls activation of adipose tissue thermogenesis
 Light on leptin link to lipolysis
Light on leptin link to lipolysis
 Gut feeling for food choice
Gut feeling for food choice