Abstract
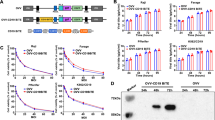
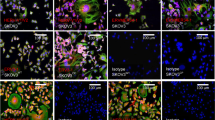
Sindbis viral vectors target and inhibit the growth of various solid tumors in mouse models. However, their efficacy against blood cancer has not been well established. Here, we show that Sindbis vectors infect and efficiently trigger apoptosis in mouse BW5147 malignant hematopoietic T-cells, but only at low levels in human lymphoma and leukemia cells (Jurkat, Karpas, CEM, DHL and JB). The Mr 37/67 kD laminin receptor (LAMR) has been suggested to be the receptor for Sindbis virus. However, JB cells, which are infected by Sindbis at low efficiency, express high levels of LAMR, revealing that additional factors are involved in Sindbis tropism. To test the infectivity and therapeutic efficacy of Sindbis vectors against malignant hematopoietic cells in vivo, we injected BW5147 cells intraperitoneally into (C3HXAKR) F1 hybrid mice. We found that Sindbis vectors targeted the tumors and significantly prolonged survival of tumor-bearing mice. We also tested the Sindbis vectors in a transgenic CD4-Rgr model, which spontaneously develop thymic lymphomas. However, infectivity in this model was less efficient. Taken together, these results demonstrate that Sindbis vectors have the potential to target and kill hematopoietic malignancies in mice, but further research is needed to evaluate the mechanism underlining the susceptibility of human lymphoid malignancies to Sindbis therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Howlader NNA, Krapcho M . Cancer satatistics & reviews. NCI. Available at: http://www.lls.org/#/diseaseinformation/getinformationsupport/factsstatistics/ Accessed 29 March 2012.
Bredenbeek PJ, Frolov I, Rice CM, Schlesinger S . Sindbis virus expression vectors: packaging of RNA replicons by using defective helper RNAs. J Virol 1993; 67: 6439–6446.
Strauss JH, Strauss SE . The alphaviruses: gene expression, relication, and evolution. Microbiol Rev 1994; 58: 72.
Altman-Hamamdzic S, Groseclose C, Ma JX, Hamamdzic D, Vrindavanam NS, Middaugh LD et al. Expression of beta-galactosidase in mouse brain: utilization of a novel nonreplicative Sindbis virus vector as a neuronal gene delivery system. Gene Ther 1997; 4: 815–822.
Gwag BJ, Kim EY, Ryu BR, Won SJ, Ko HW, Oh YJ et al. A neuron-specific gene transfer by a recombinant defective Sindbis virus. Brain Res Mol Brain Res 1998; 63: 53–61.
Tsuji M, Bergmann CC, Takita-Sonoda Y, Murata K, Rodrigues EG, Nussenzweig RS et al. Recombinant Sindbis viruses expressing a cytotoxic T-lymphocyte epitope of a malaria parasite or of influenza virus elicit protection against the corresponding pathogen in mice. J Virol 1998; 72: 6907–6910.
Pugachev KV, Mason PW, Shope RE, Frey TK . Double-subgenomic Sindbis virus recombinants expressing immunogenic proteins of Japanese encephalitis virus induce significant protection in mice against lethal JEV infection. Virology 1995; 212: 587–594.
Hariharan MJ, Driver DA, Townsend K, Brumm D, Polo JM, Belli BA et al. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol 1998; 72: 950–958.
Turrell MJ . Horizontal and vertical transmission of viruses by insect by and tick vectors. CRC Press Inc: Boca Raton (FL), 1988.
Ryman KD, Klimstra WB, Nguyen KB, Biron CA, Johnston RE . Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J Virol 2000; 74: 3366–3378.
Cook SH, Griffin DE . Luciferase imaging of a neurotropic viral infection in intact animals. J Virol 2003; 77: 5333–5338.
Postic B, Schleupner CJ, Armstrong JA, Ho M . Two variants of Sindbis virus which differ in interferon induction and serum clearance. I. The phenomenon. J Infect Dis 1969; 120: 339–347.
Jan JT, Griffin DE . Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J Virol 1999; 73: 10296–10302.
Jan JT, Chatterjee S, Griffin DE . Sindbis virus entry into cells triggers apoptosis by activating sphingomyelinase, leading to the release of ceramide. J Virol 2000; 74: 6425–6432.
Xiong C, Levis R, Shen P, Schlesinger S, Rice CM, Huang HV . Sindbis virus: an efficient, broad host range vector for gene expression in animal cells. Science (New York, NY) 1989; 243: 1188–1191.
Balachandran S, Roberts PC, Kipperman T, Bhalla KN, Compans RW, Archer DR et al. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J Virol 2000; 74: 1513–1523.
Hurtado A, Tseng JC, Meruelo D . Gene therapy that safely targets and kills tumor cells throughout the body. Rejuvenation Res 2006; 9: 36–44.
Wang KS, Kuhn RJ, Strauss EG, Ou S, Strauss JH . High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J Virol 1992; 66: 4992–5001.
Levine B, Huang Q, Isaacs JT, Reed JC, Griffin DE, Hardwick JM . Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 1993; 361: 739–742.
Tseng JC, Hurtado A, Yee H, Levin B, Boivin C, Benet M et al. Using sindbis viral vectors for specific detection and suppression of advanced ovarian cancer in animal models. Cancer Res 2004; 64: 6684–6692.
Menard S, Tagliabue E, Colnaghi MI . The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res Treat 1998; 52: 137–145.
Viacava P, Naccarato AG, Collecchi P, Menard S, Castronovo V, Bevilacqua G . The spectrum of 67-kD laminin receptor expression in breast carcinoma progression. J Pathol 1997; 182: 36–44.
Martignone S, Menard S, Bufalino R, Cascinelli N, Pellegrini R, Tagliabue E et al. Prognostic significance of the 67-kilodalton laminin receptor expression in human breast carcinomas. J Natl Cancer Inst 1993; 85: 398–402.
Basolo F, Pollina L, Pacini F, Fontanini G, Menard S, Castronovo V et al. Expression of the Mr 67,000 laminin receptor is an adverse prognostic indicator in human thyroid cancer: an immunohistochemical study. Clin Cancer Res 1996; 2: 1777–1780.
Sanjuan X, Fernandez PL, Miquel R, Munoz J, Castronovo V, Menard S et al. Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. J Pathol 1996; 179: 376–380.
Cioce V, Castronovo V, Shmookler BM, Garbisa S, Grigioni WF, Liotta LA et al. Increased expression of the laminin receptor in human colon cancer. J Natl Cancer Inst 1991; 83: 29–36.
de Manzoni G, Verlato G, Tomezzoli A, Guglielmi A, Pelosi G, Ricci F et al. Study on Ki-67 immunoreactivity as a prognostic indicator in patients with advanced gastric cancer. Jpn J Clin Oncol 1998; 28: 534–537.
Pelosi G, Pasini F, Bresaola E, Bogina G, Pederzoli P, Biolo S et al. High-affinity monomeric 67-kD laminin receptors and prognosis in pancreatic endocrine tumours. J Pathol 1997; 183: 62–69.
Tarbe N, Evtimova V, Burtscher H, Jarsch M, Alves F, Weidle UH . Transcriptional profiling of cell lines derived from an orthotopic pancreatic tumor model reveals metastasis-associated genes. Anticancer Res 2001; 21: 3221–3228.
van den Brule FA, Castronovo V, Menard S, Giavazzi R, Marzola M, Belotti D et al. Expression of the 67 kD laminin receptor in human ovarian carcinomas as defined by a monoclonal antibody, MLuC5. Eur J Cancer 1996; 32A: 1598–1602.
Taraboletti G, Belotti D, Giavazzi R, Sobel ME, Castronovo V . Enhancement of metastatic potential of murine and human melanoma cells by laminin receptor peptide G: attachment of cancer cells to subendothelial matrix as a pathway for hematogenous metastasis. J Natl Cancer Inst 1993; 85: 235–240.
Ozaki I, Yamamoto K, Mizuta T, Kajihara S, Fukushima N, Setoguchi Y et al. Differential expression of laminin receptors in human hepatocellular carcinoma. Gut 1998; 43: 837–842.
van den Brule FA, Buicu C, Berchuck A, Bast RC, Deprez M, Liu FT et al. Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Hum Pathol 1996; 27: 1185–1191.
Siegel S, Wagner A, Kabelitz D, Marget M, Coggin J, Barsoum A et al. Induction of cytotoxic T-cell responses against the oncofetal antigen-immature laminin receptor for the treatment of hematologic malignancies. Blood 2003; 102: 4416–4423.
Tseng JC, Levin B, Hurtado A, Yee H, Perez de Castro I, Jimenez M et al. Systemic tumor targeting and killing by Sindbis viral vectors. Nat Biotechnol 2004; 22: 70–77.
Tseng JC, Levin B, Hirano T, Yee H, Pampeno C, Meruelo D . In vivo antitumor activity of Sindbis viral vectors. J Natl Cancer Inst 2002; 94: 1790–1802.
Tan L, Xu B, Liu R, Liu H, Tan H, Huang W . Gene therapy for acute myeloid leukemia using Sindbis vectors expressing a fusogenic membrane glycoprotein. Cancer Biol Ther 2010; 9: 350–357.
Krishnamurthy S, Takimoto T, Scroggs RA, Portner A . Differentially regulated interferon response determines the outcome of Newcastle disease virus infection in normal and tumor cell lines. J Virol 2006; 80: 5145–5155.
Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 2000; 6: 821–825.
Parato KA, Senger D, Forsyth PA, Bell JC . Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer 2005; 5: 965–976.
Huang PY, Guo JH, Hwang LH . Oncolytic Sindbis virus targets tumors defective in the interferon response and induces significant bystander antitumor immunity in vivo. Mol Ther 2012; 20: 298–305.
Jimenez M, Perez de Castro I, Benet M, Garcia JF, Inghirami G, Pellicer A . The Rgr oncogene induces tumorigenesis in transgenic mice. Cancer Res 2004; 64: 6041–6049.
Scheiman J, Tseng JC, Zheng Y, Meruelo D . Multiple functions of the 37/67-kd laminin receptor make it a suitable target for novel cancer gene therapy. Mol Ther 2010; 18: 63–74.
Osei-Sarfo K, Martello L, Ibrahim S, Pellicer A . The human Rgr oncogene is overexpressed in T-cell malignancies and induces transformation by acting as a GEF for Ras and Ral. Oncogene 2011; 30: 3661–3671.
Zalman MA, Meruelo D . Analysis of H-2-linked immune responses involved in resistance to AKR tumor growth. Immunogenetics 1986; 24: 51–62.
Montuori N, Selleri C, Risitano AM, Raiola AM, Ragno P, Del Vecchio L et al. Expression of the 67-kDa laminin receptor in acute myeloid leukemia cells mediates adhesion to laminin and is frequently associated with monocytic differentiation. Clin Cancer Res 1999; 5: 1465–1472.
Wang L, Zhu J, Shan S, Qin Y, Kong Y, Liu J et al. Repression of interferon-gamma expression in T cells by Prospero-related homeobox protein. Cell Res 2008; 18: 911–920.
Lee WH, Liu FH, Lee YL, Huang HM . Interferon-alpha induces the growth inhibition of human T-cell leukaemia line Jurkat through p38alpha and p38beta. J Biochem 2010; 147: 645–650.
Stevens CN, Simeone AM, John S, Ahmed Z, Lucherini OM, Baldari CT et al. T-cell receptor early signalling complex activation in response to interferon-alpha receptor stimulation. Biochem J 2010; 428: 429–437.
Willers J, Dummer R, Kempf W, Kundig T, Burg G, Kadin ME . Proliferation of CD30+ T-helper 2 lymphoma cells can be inhibited by CD30 receptor cross-linking with recombinant CD30 ligand. Clin Cancer Res 2003; 9: 2744–2754.
Dupont SA, Goelz S, Goyal J, Green M . Mechanisms for regulation of cellular responsiveness to human IFN-beta1a. J Interferon Cytokine Res 2002; 22: 491–501.
Pasternack MS, Bevan MJ, Klein JR . Release of discrete interferons by cytotoxic T lymphocytes in response to immune and nonimmune stimuli. J Immunol 1984; 133: 277–280.
Vieillard V, Lauret E, Rousseau V, De Maeyer E . Blocking of retroviral infection at a step prior to reverse transcription in cells transformed to constitutively express interferon beta. Proc Natl Acad Sci USA 1994; 91: 2689–2693.
Espert L, Degols G, Lin YL, Vincent T, Benkirane M, Mechti N . Interferon-induced exonuclease ISG20 exhibits an antiviral activity against human immunodeficiency virus type 1. J Gen Virol 2005; 86 (Pt 8): 2221–2229.
Acknowledgements
We thank Dr Tomer Granot and Ms Susanne Tranguch for the critical reading of the manuscript and helpful discussions. This study was supported by NIH CA50434 and Lymphoma & Leukemia Society 6064-07 grants. All animal experiments were performed in both the animal facility of NYU Langone Medical Center and of the Joan and Joel Smilow research center according to the NIH guidelines (Guide for the care and use of the Laboratory Animals, 1996).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Cancer Gene Therapy website
Rights and permissions
About this article
Cite this article
Suzme, R., Tseng, JC., Levin, B. et al. Sindbis viral vectors target hematopoietic malignant cells. Cancer Gene Ther 19, 757–766 (2012). https://doi.org/10.1038/cgt.2012.56
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.56
Keywords
This article is cited by
-
Gene and virotherapy for hematological malignancies
International Journal of Hematology (2016)