Abstract
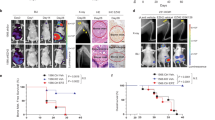
Breast cancer metastasis to bone represents a devastating complication of advanced breast cancer, frequently resulting in significant increases in morbidity and mortality. An understanding of the mechanisms that govern breast cancer metastasis at the molecular level should lead to more effective therapies. Recently, the kringle 1 domain of human hepatocyte growth factor (HGFK1) was identified as a candidate metastasis suppressor gene. Here, we investigated whether HGFK1 is a key regulator of breast cancer bone metastasis. Of the 193 human breast carcinoma tissue samples examined, HGFK1 expression was relative higher in 82 (42.4%) by western blot and in 84 (43.5%) by quantitative real-time PCR. The higher expression of HGFK1 was significantly associated with a better prognostic value (P<0.001) and inversely correlated with bone metastasis (P=0.003). The efficacy of adeno-associated virus carrying HGFK1 (AAV-HGFK1) in osteolytic bone metastasis was then evaluated using an in vivo bone metastasis model. AAV-HGFK1 significantly inhibited osteolytic bone metastasis and prolonged the survival of mice in this model (P<0.01). In vitro, HGFK1 expression resulted in significant anti-invasion effects, enhanced the phosphorylation of TAK1 (transforming growth factor-β-activated kinase 1), p38 MAPK (mitogen-activated protein kinase) and MAPKAPK2 (MAPK-activated protein kinase 2) and decreased the expression of receptor activator of nuclear factor-κB (RANK), which was abrogated by the p38 MAPK inhibitor SB203580. This study shows for the first time that HGFK1 significantly inhibits the metastasis of breast cancer to bone by activating the TAK1/p38 MAPK signaling pathway and inhibiting RANK expression. Thus, AAV-HGFK1 treatment represents a potential therapy for bone metastasis in breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mundy GR . Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593.
Kozlow W, Guise TA . Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia 2005; 10: 169–180.
Roodman GD . Mechanisms of bone metastasis. N Engl J Med 2004; 350: 1655–1664.
Wittrant Y, Theoleyre S, Chipoy C, Padrines M, Blanchard F, Heymann D et al. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta 2004; 1704: 49–57.
Blair JM, Zhou H, Seibel MJ, Dunstan CR . Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat Clin Pract Oncol 2006; 3: 41–49.
Baud’huin M, Duplomb L, Ruiz Velasco C, Fortun Y, Heymann D, Padrines M . Key roles of the OPG-RANK-RANKL system in bone oncology. Expert Rev Anticancer Ther 2007; 7: 221–232.
Dougall WC, Chaisson M . The RANK/RANKL/OPG triad in cancer-induced bone diseases. Cancer Metastasis Rev 2006; 25: 541–549.
Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 2006; 440: 692–696.
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997; 89: 309–319.
Xin L, Xu R, Zhang Q, Li TP, Gan RB . Kringle 1 of human hepatocyte growth factor inhibits bovine aortic endothelial cell proliferation stimulated by basic fibroblast growth factor and causes cell apoptosis. Biochem Biophys Res Commun 2000; 277: 186–190.
Shen Z, Yang ZF, Gao Y, Li JC, Chen HX, Liu CC et al. The kringle 1 domain of hepatocyte growth factor has antiangiogenic and antitumor cell effects on hepatocellular carcinoma. Cancer Res 2008; 68: 404–414.
Zhu J, Jia X, Xiao G, Kang Y, Partridge NC, Qin L . EGF-like ligands stimulate osteoclastogenesis by regulating expression of osteoclast regulatory factors by osteoblasts: implications for osteolytic bone metastases. J Biol Chem 2007; 282: 26656–26664.
Chen Y, Luk KD, Cheung KM, Xu R, Lin MC, Lu WW et al. Gene therapy for new bone formation using adeno-associated viral bone morphogenetic protein-2 vectors. Gene Therapy 2003; 10: 1345–1353.
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res 2006; 12: 5369–5376.
Rachner TD, Khosla S, Hofbauer LC . Osteoporosis: now and the future. Lancet 2011; 377: 1276–1287.
Singh AS, Figg WD . In vivo models of prostate cancer metastasis to bone. J Urol 2005; 174: 820–826.
Ponnazhagan S, Mahendra G, Kumar S, Shaw DR, Stockard CR, Grizzle WE et al. Adeno-associated virus 2-mediated antiangiogenic cancer gene therapy: long-term efficacy of a vector encoding angiostatin and endostatin over vectors encoding a single factor. Cancer Res 2004; 64: 1781–1787.
Subramanian IV, Bui Nguyen TM, Truskinovsky AM, Tolar J, Blazar BR, Ramakrishnan S . Adeno-associated virus-mediated delivery of a mutant endostatin in combination with carboplatin treatment inhibits orthotopic growth of ovarian cancer and improves long-term survival. Cancer Res 2006; 66: 4319–4328.
Wendt MK, Smith JA, Schiemann WP . Transforming growth factor-beta-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene 2010; 29: 6485–6498.
Yu L, Hebert MC, Zhang YE . TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J 2002; 21: 3749–3759.
Tchaikovski V, Fellbrich G, Waltenberger J . The molecular basis of VEGFR-1 signal transduction pathways in primary human monocytes. Arterioscler Thromb Vasc Biol 2008; 28: 322–328.
Santini D, Schiavon G, Vincenzi B, Gaeta L, Pantano F, Russo A et al. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS One 2011; 6: e19234.
Acknowledgements
We are thankful for financial support from National Natural Science Foundation of China (No. 30872991), Natural Science Foundation of Zhejiang Province (Y2007048) and Science and technology project of Zhejiang Province (2009C33SA800006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yao, Y., Fang, ZP., Chen, H. et al. HGFK1 inhibits bone metastasis in breast cancer through the TAK1/p38 MAPK signaling pathway. Cancer Gene Ther 19, 601–608 (2012). https://doi.org/10.1038/cgt.2012.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2012.38
Keywords
This article is cited by
-
Combination of Peglated-H1/HGFK1 Nanoparticles and TAE in the Treatment of Hepatocellular Carcinoma
Applied Biochemistry and Biotechnology (2023)
-
Demethoxycucumin protects MDA-MB-231 cells induced bone destruction through JNK and ERK pathways inhibition
Cancer Chemotherapy and Pharmacology (2021)
-
Peglated-H1/pHGFK1 nanoparticles enhance anti-tumor effects of sorafenib by inhibition of drug-induced autophagy and stemness in renal cell carcinoma
Journal of Experimental & Clinical Cancer Research (2019)
-
TAK1 mediates microenvironment-triggered autocrine signals and promotes triple-negative breast cancer lung metastasis
Nature Communications (2018)
-
Prevalence and risk factors of bone metastasis and skeletal related events in patients with primary breast cancer in Japan
International Journal of Clinical Oncology (2014)