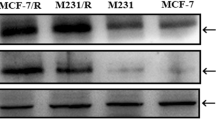
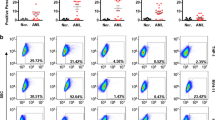
Abstract
Oncolytic virus-armed gene therapy may offer new treatment options and improve the prognosis for patients with colon cancer. In this study, we sought to further confirm the antitumor activity of oncolytic virus-armed tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene therapy in xenografts, which derived from the tumors of patients with colon cancer. To this end, we established xenotransplantable tumors from fresh surgical specimens. The histology of these xenografts maintained the features of the original tumors during passaging in nude mice. We next treated these xenografts with adenoviruses carrying TRAIL and the adenovirus E1A gene (Ad/TRAIL-E1) driven by the human telomerase reverse transcriptase promoter. The vector expressing the TRAIL gene (Ad/gTRAIL) or the E1A gene (Ad/GFP-E1) alone was used as control vector. The results demonstrated that Ad/TRAIL-E1 had more significant inhibitory effects on tumor growth than Ad/gTRAIL or Ad/GFP-E1 alone. Furthermore, we did not find any obvious treatment-related toxicity in the mice. Our results indicate that the use of an oncolytic adenoviral vector, in combination with TRAIL gene therapy, is a promising novel approach for cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P . Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108.
Wolpin BM, Meyerhardt JA, Mamon HJ, Mayer RJ . Adjuvant treatment of colorectal cancer. CA Cancer J Clin 2007; 57: 168–185.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96.
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995; 3: 673–682.
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A . Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996; 271: 12687–12690.
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999; 104: 155–162.
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5: 157–163.
French LE, Tschopp J . The TRAIL to selective tumor death. Nat Med 1999; 5: 146–147.
Gliniak B, Le T . Tumor necrosis factor-related apoptosis-inducing ligand's antitumor activity in vivo is enhanced by the chemotherapeutic agent CPT-11. Cancer Res 1999; 59: 6153–6158.
Chinnaiyan AM, Prasad U, Shankar S, Hamstra DA, Shanaiah M, Chenevert TL et al. Combined effect of tumor necrosis factor-related apoptosis-inducing ligand and ionizing radiation in breast cancer therapy. Proc Natl Acad Sci USA 2000; 97: 1754–1759.
LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P et al. Tumor-cell resistance to death receptor—induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med 2002; 8: 274–281.
Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH . ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med 1997; 3: 639–645.
Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 1996; 274: 373–376.
Garber K . China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst 2006; 98: 298–300.
van Beusechem VW, van den Doel PB, Grill J, Pinedo HM, Gerritsen WR . Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res 2002; 62: 6165–6171.
Wadler S, Yu B, Tan JY, Kaleya R, Rozenblit A, Makower D et al. Persistent replication of the modified chimeric adenovirus ONYX-015 in both tumor and stromal cells from a patient with gall bladder carcinoma implants. Clin Cancer Res 2003; 9: 33–43.
Hann B, Balmain A . Replication of an E1B 55-kilodalton protein-deficient adenovirus (ONYX-015) is restored by gain-of-function rather than loss-of-function p53 p53 mutants. J Virol 2003; 77: 11588–11595.
Dong F, Wang L, Davis JJ, Hu W, Zhang L, Guo W et al. Eliminating established tumor in nu/nu nude mice by a tumor necrosis factor-alpha-related apoptosis-inducing ligand-armed oncolytic adenovirus. Clin Cancer Res 2006; 12: 5224–5230.
Davis JJ, Wang L, Dong F, Zhang L, Guo W, Teraishi F et al. Oncolysis and suppression of tumor growth by a GFP-expressing oncolytic adenovirus controlled by an hTERT and CMV hybrid promoter. Cancer Gene Ther 2006; 13: 720–723.
Lin T, Gu J, Zhang L, Huang X, Stephens LC, Curley SA et al. Targeted expression of green fluorescent protein/tumor necrosis factor-related apoptosis-inducing ligand fusion protein from human telomerase reverse transcriptase promoter elicits antitumor activity without toxic effects on primary human hepatocytes. Cancer Res 2002; 62: 3620–3625.
Jacob D, Davis J, Zhu H, Zhang L, Teraishi F, Wu S et al. Suppressing orthotopic pancreatic tumor growth with a fiber-modified adenovector expressing the TRAIL gene from the human telomerase reverse transcriptase promoter. Clin Cancer Res 2004; 10: 3535–3541.
Gu J, Kagawa S, Takakura M, Kyo S, Inoue M, Roth JA et al. Tumor-specific transgene expression from the human telomerase reverse transcriptase promoter enables targeting of the therapeutic effects of the Bax gene to cancers. Cancer Res 2000; 60: 5359–5364.
Fang B, Ji L, Bouvet M, Roth JA . Evaluation of GAL4/TATA in vivo. Induction of transgene expression by adenovirally mediated gene codelivery. J Biol Chem 1998; 273: 4972–4975.
Giovanella BC, Yim SO, Stehlin JS, Williams Jr LJ . Development of invasive tumors in the ‘nude’ mouse after injection of cultured human melanoma cells. J Natl Cancer Inst 1972; 48: 1531–1533.
Bosma GC, Custer RP, Bosma MJ . A severe combined immunodeficiency mutation in the mouse. Nature 1983; 301: 527–530.
Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer 2001; 84: 1424–1431.
Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de CP et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res 2007; 13: 3989–3998.
Bhowmick NA, Neilson E, GMoses HL . Stromal fibroblasts in cancer initiation and progression. Nature 2004; 432: 332–337.
Albini A, Sporn MB . The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 2007; 7: 139–147.
Fichtner I, Slisow W, Gill J, Becker M, Elbe B, Hillebrand T et al. Anticancer drug response and expression of molecular markers in early-passage xenotransplanted colon carcinomas. Eur J Cancer 2004; 40: 298–307.
Peterson JK, Houghton PJ . Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer 2004; 40: 837–844.
Fiebig HH, Maier A, Burger AM . Clonogenic assay with established human tumour xenografts: correlation of in vitro to in vivo activity as a basis for anticancer drug discovery. Eur J Cancer 2004; 40: 802–820.
Judde JG, Rebucci M, Vogt N, de Cremoux P, Livartowski A, Chapelier A et al. Gefitinib and chemotherapy combination studies in five novel human non small cell lung cancer xenografts. Evidence linking EGFR signaling to gefitinib antitumor response. Int J Cancer 2007; 120: 1579–1590.
Fichtner I, Rolff J, Soong R, Hoffmann J, Hammer S, Sommer A et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res 2008; 14: 6456–6468.
Mi J, Li ZY, Ni S, Steinwaerder D, Lieber A . Induced apoptosis supports spread of adenovirus vectors in tumors. Hum Gene Ther 2001; 12: 1343–1352.
Solly SK, Trajcevski S, Frisen C, Holzer GW, Nelson E, Clerc B et al. Replicative retroviral vectors for cancer gene therapy. Cancer Gene Ther 2003; 10: 30–39.
Pulkkanen KJ, Yla-Herttuala S . Gene therapy for malignant glioma: current clinical status. Mol Ther 2005; 12: 585–598.
Colombo F, Barzon L, Franchin E, Pacenti M, Pinna V, Danieli D et al. Combined HSV-TK/IL-2 gene therapy in patients with recurrent glioblastoma multiforme: biological and clinical results. Cancer Gene Ther 2005; 12: 835–848.
Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE . Oncolytic viruses in cancer therapy. Cancer Lett 2007; 254: 178–216.
Shinoura N, Yoshida Y, Asai A, Kirino T, Hamada H . Adenovirus-mediated transfer of p53 and Fas ligand drastically enhances apoptosis in gliomas. Cancer Gene Ther 2000; 7: 732–738.
Shao R, Lee DF, Wen Y, Ding Y, Xia W, Ping B et al. E1A sensitizes cancer cells to TRAIL-induced apoptosis through enhancement of caspase activation. Mol Cancer Res 2005; 3: 219–226.
Acknowledgements
We thank Yongqiang Liao for expert technical assistance in histology. This work was supported in part by grants from the National Natural Science Foundation of China grant 30528030 and 30700970. This article represents a partial fulfillment of the requirements for a Ph.D. degree for Wei Zhou.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhou, W., Zhu, H., Chen, W. et al. Treatment of patient tumor-derived colon cancer xenografts by a TRAIL gene-armed oncolytic adenovirus. Cancer Gene Ther 18, 336–345 (2011). https://doi.org/10.1038/cgt.2010.83
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.83
Keywords
This article is cited by
-
Immuno-Oncolytic Viruses: Emerging Options in the Treatment of Colorectal Cancer
Molecular Diagnosis & Therapy (2021)
-
Telomerase-specific oncolytic adenovirus expressing TRAIL suppresses peritoneal dissemination of gastric cancer
Gene Therapy (2017)
-
Enhancing the bystander killing effect of an oncolytic HSV by arming it with a secretable apoptosis activator
Gene Therapy (2015)