Abstract
Programmed cell death 5 (PDCD5) is an apoptosis promoter molecule that displays multiple biological activities. However, the function of PDCD5 in vivo has not yet been investigated. Here, we generated a Pdcd5 knockout mouse model to study the physiological role of PDCD5 in vivo. Knockout of the Pdcd5 gene resulted in embryonic lethality at mid-gestation. Histopathological analysis revealed dysplasia in both the LZs and JZs in Pdcd5–/– placentas with defects in spongiotrophoblasts and trophoblast giant cells. Furthermore, Pdcd5–/– embryos had impaired transplacental passage capacity. We also found that Pdcd5–/– embryos exhibited cardiac abnormalities and defective liver development. The growth defect is linked to impaired placental development and may be caused by insufficient oxygen and nutrient transfer across the placenta. These findings were verified in vitro in Pdcd5 knockout mouse embryonic fibroblasts, which showed increased apoptosis and G0/G1 phase cell cycle arrest. Pdcd5 knockout decreased the Vegf and hepatocyte growth factor (Hgf) levels, downregulated the downstream Pik3ca–Akt–Mtor signal pathway and decreased cell survival. Collectively, our studies demonstrated that Pdcd5 knockout in mouse embryos results in placental defects and embryonic lethality.
Similar content being viewed by others
Main
The PDCD5 (programmed cell death 5) gene, formerly designated as TFAR19 (TF-1 cell apoptosis-related gene-19), was first discovered in 1999.1 Existing studies on PDCD5 have revealed that its expression is downregulated in a variety of tumor tissues, and PDCD5 overexpression can promote apoptosis in different tumor cell types in response to various stimuli,1, 2, 3 as well as participate in other types of cell death such as paraptosis4 and autophagy.5 Studies have revealed that PDCD5 can interact with multiple molecules such as TIP60,3 TP53,6, 7 MDM26 and HDAC3,7 and PDCD5 has been shown to play an important role in the TIP60/HDAC3-TP53 signaling pathway to promote apoptosis and G0/G1 phase cell cycle arrest. PDCD5 can also interact with CCT8 to inhibit β-tubulin folding and influence cell division and proliferation.
PDCD5 expression in cells is finely regulated. Transcription factor NF-κB p65 promotes the levels of PDCD5 mRNA and transcriptional activity.9 OTU deubiquitinase 5 (OTUD5) and YY1-associated factor 2 (YAF2) interact with PDCD5 to enhance its stability and inhibit proteasome-dependent degradation.10, 11 In contrast, DNAJB1 enhances ubiquitination and degradation of PDCD5.12
Recently, we found that PDCD5 interacts with FOXP3, increases acetylation of FOXP3 in synergy with TIP60 and enhances the repressive function of FOXP3.13 In PDCD5 transgenic (PDCD5tg) mice, PDCD5 overexpression could alleviate the severity of experimentally induced autoimmune encephalomyelitis (EAE).13 In a rat collagen-induced arthritis model, rats that were treated with recombinant human PDCD5 protein significantly delayed the occurrence and reduced the severity of arthritis.14 Moreover, rhPDCD5 also exerted anti-inflammatory effects in EAE mice.15 Additionally, PDCD5 may also play an important role in influenza virus (H1N1) infection,16 cerebral ischemia/reperfusion injury17 and cardiac remodeling,5 indicating the diversity and complexity of its functions.
Thus far, however, the function of Pdcd5 in vivo remains totally unknown. Therefore, in the present study, we generated Pdcd5 knockout (Pdcd5–/–) mice to study the physiological role of Pdcd5 in vivo.
Results
Pdcd5 knockout in mice leads to embryonic lethality
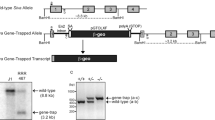
Using Zp3-Cre transgenic mice, in which Pdcd5 is exclusively expressed in growing oocytes under the control of the zona pellucida glycoprotein 3 promoter,18 we obtained heterozygous Pdcd5 (Pdcd5+/–) mutant mice with deleted exons 2 and 3. Figure 1a is a schematic representation of the mouse Pdcd5 genomic structure, the LoxP-modified Pdcd5 loci and the mutant Pdcd5 structure. Long-term observation revealed that Pdcd5+/− mice appeared phenotypically normal, and the eldest mouse survived beyond 15 months of age without evidence of significant spontaneous disease. Theoretically, Pdcd5–/– mice can be generated by the intercrossing of Pdcd5+/− mice. However, genotyping of more than 100 pups identified no homozygous mice (Figure 1b), whereas Pdcd5+/+ and Pdcd5+/−pups had a Mendelian ratio of nearly 1:2 (Supplementary Table S1). These results indicated that pups homozygous for Pdcd5–/− died in utero.
Pdcd5 knockout mice exhibited intrauterine growth restriction and embryonic lethality. (a) Schematic representation of the mouse Pdcd5 genomic structure, the LoxP-modified Pdcd5 loci and the mutant Pdcd5 structure. Boxes represent the six Pdcd5 exons. The triangles represent the LoxP sites, and primers P1, P2 and P3 are indicated for PCR genotyping. (b and c) Representative PCR results for genotyping of postnatal mice (b) and E12.5 embryos (c) derived from intercrossing between Pdcd5+/– mice. (d) The relative Pdcd5 mRNA levels were analyzed in Pdcd5+/+, Pdcd5−/+ and Pdcd5–/– embryos by RT-PCR. (e) The expression of Pdcd5 protein in Pdcd5+/+ and Pdcd5–/–embryos detected by western blot analysis. (f–h) Representative images of Pdcd5+/+ and Pdcd5–/– embryos at E15.5 (f), E13.5 (g) and E11.5 (h). (i) Pdcd5+/+ and Pdcd5–/–embryos at E13.5 were analyzed by hematoxylin and eosin (H&E) staining. (j) Representative images of Pdcd5+/+ and Pdcd5–/–placentas at E11.5 and E13.5
To identify the time of death, embryos derived from heterozygous intercrosses were genotyped at various stages of gestation. The results of the genotype and phenotype analysis are summarized in Supplementary Table S1. As shown in the table, no viable Pdcd5–/– embryos were recovered after embryonic day 13.5 (E13.5). Representative PCR identification of mouse embryos at E12.5 is shown in Figure 1c. Results of the RT-PCR and western blot analyses demonstrated that normal Pdcd5 mRNA and Pdcd5 protein were undetectable in Pdcd5–/– embryos (Figures 1d and e). At E15.5, the Pdcd5–/– embryos were obviously dead and resorbed (Figure 1f), and we could not detect any live Pdcd5−/−embryos at E15.5. Although visible homozygous embryos could still be detected at E13.5, they appeared soft and pale, and could not maintain their shape (Figure 1g). Histological analysis of embryos obtained at E13.5 showed that Pdcd5–/– embryos had undergone complete autolysis, and none of the organs could be identified (Figure 1i), suggesting that the point of lethality for the homozygous embryos was approximately E13.5. At E11.5, Pdcd5–/– embryos were also smaller and paler than Pdcd5+/+ embryos (Figure 1h). At E12.5 embryos, the architecture of mutant embryos appeared normal, but the overall shape of these embryos was smaller than that of the wild-type (WT) embryos (Supplementary Figure S1). These results showed that homozygous deletion of Pdcd5 led to growth retardation and embryonic lethality at mid-gestation, suggesting that Pdcd5 is indispensable in fetal development.
Impaired development of junctional and labyrinth zones in Pdcd5–/– placentas
Gross examination of the placenta revealed that the Pdcd5–/– placentas were smaller than the Pdcd5+/+ placentas (Figure 1j); therefore, we performed histological analysis of the placental tissues. As shown in Figures 2a–c, Pdcd5+/+ placentas showed normal architecture with three compartments: maternal decidua basalis (DB), junctional zone (JZ) and labyrinth zone (LZ) where nutrient transfer occurs between the maternal and fetal blood spaces.19 However, LZ and JZ exhibited a drastic reduction in thickness in the Pdcd5–/–placenta (Figures 2a–c, left panel). At E13.5, the mutant placentas showed disordered structure, hemorrhage and necrosis compared with Pdcd5+/+ placentas (Figure 2c). At a higher magnification, we observed that the number of invading maternal blood sinuses in Pdcd5–/– LZ was strongly decreased compared with that in normal LZ (Figure 2d). The mutant LZ did not have the highly vascularized organization and appeared to be in a compact state. There was no obvious difference in the number of fetal blood vessels (identified by the nucleated embryonic blood cells within them) between Pdcd5–/– and Pdcd5+/+ LZ. Accordingly, the ratio of maternal blood vessels to fetal blood vessels was reduced in mutant LZ (Figure 2e). Ultrastructure analysis of the LZ (Figure 3a) revealed a disordered alignment of blood vessels in the Pdcd5–/– LZ, indicating that the LZ was markedly underdeveloped in Pdcd5–/– placentas.
Developmental defects in the labyrinth and junctional zones in Pdcd5–/– placentas. (a–c) H&E staining of Pdcd5+/+ and Pdcd5–/– placentas at E10.5 (a), E12.5 (b) and E13.5 (c). The layers of the placental architecture are labeled: decidua basalis (DB), junctional zone (JZ) and labyrinth zone (LZ). (d) H&E staining of the LZ of Pdcd5+/+ and Pdcd5–/– placentas at E12.5. Vessels were separated by erythrocytes of maternal blood sinuses (asterisks) and fetal vessels (arrows). (e) Histogram shows the ratio of maternal vessels/fetus from Pdcd5+/+ and Pdcd5–/– LZ at E10.5 and E12.5. Three placentas from each genotype and five different sections from each placenta were analyzed. Data are means±S.D. ***P<0.001. (f) H&E staining of the JZ of Pdcd5+/+ and Pdcd5–/– placentas at E12.5. (g and h) Histogram shows the number (g) and nucleus size (h) of TGC from Pdcd5+/+ and Pdcd5–/– JZ at E10.5 and E12.5. Three placentas from each genotype and five different sections from each placenta were analyzed. Data are means±S.D. ***P<0.001, **P<0.01
Analysis of Pdcd5+/+ and Pdcd5–/– placentas by scanning electron microscopy and PAS staining. (a) Scanning electron microscope analysis of the labyrinth zone in Pdcd5+/+ and Pdcd5–/– placentas at E12.5 (× 10 000). (b) Periodic acid/Schiff (PAS) staining of Pdcd5+/+ and Pdcd5–/– placentas at E12.5. The layers of the placental architecture are labeled: decidua basalis (DB), junctional zone (JZ) and labyrinth zone (LZ). (c and d) PAS staining in Pdcd5+/+ and Pdcd5−/− LZ and JZ at E12.5. Trophoblasts are marked as trophoblast giant cells (asterisks), glycogen trophoblasts (triangles) and spongiotrophoblasts (hearts)
The JZ is composed of spongiotrophoblasts (SpTs), glycogen trophoblasts (GlyTCs) and trophoblast giant cells (TGCs). The TGCs are located at the uterine trophoblastic border and play a role in endocrine function. As illustrated in Figures 2f–h, the number and size of TGCs were lower in the Pdcd5–/– JZ than in the Pdcd5+/+ JZ. Similarly, the number of SpTs and GlyTCs was also significantly decreased in the homozygous mutant JZ. The paraffin-sliced sections of the placentas were further stained with periodic acid/Schiff (PAS) at E12.5 (Figures 3b and c). The boundary between the JZ and LZ was more clearly visible with PAS staining than with H&E staining (Figure 3b), and the JZ and LZ were both thinner in Pdcd5−/− placentas than in Pdcd5+/+ placentas. At the same time, visualization at a higher magnification revealed a rich network structure in the Pdcd5+/+ LZ with tubular cavity (maternal blood vessels) (Figure 3c, left). However, only few maternal blood vessels were present in Pdcd5–/– LZ, which appeared very compact (Figure 3c, right). These findings were consistent with the results observed by H&E staining (Figures 2d and e). PAS-positive GlyTCs in Pdcd5+/+ JZ were stained magenta with small and dense nuclei and vacuoles in the cytoplasm. SpTs and TGCs are not stained with PAS and appear gray in the cytoplasm with large nuclei (Figure 3d, left). However, the area occupied by GlyTCs and TGCs had almost disappeared in the Pdcd5–/–JZ, and only PAS-negative SpTs were present (Figure 3d, right). Collectively, these data confirmed that the development of the LZ and JZ was significantly impaired by the absence of Pdcd5.
Placental transport is impaired in Pdcd5–/– mice
GLUT-1 is the predominant form expressed in the placenta during early pregnancy.20 Abundant GLUT-1 expression is crucial to transplacental glucose transfer via the placenta. Next, we investigated Glut-1 protein expression in the placentas at E12.5. Membrane-expressed Glut-1 was detected in both SpTs and GlyTCs but not in the TGCs of the Pdcd5+/+ JZ (Figure 4a). On the contrary, Glut-1 expression was almost absent in the JZ of Pdcd5–/– placentas because of defects in SpTs and GlyTCs (Figure 4c). There was no significant difference in the Glut-1 distribution at LZs between the two groups (Figures 4b and c). As glucose is a major nutrient passing through the placenta from the mother to the growing embryos, it is reasonable to suggest that the underdeveloped placenta may be associated with a reduction in the glucose transport capacity.
Impaired placental transport in Pdcd5–/– placentas. (a–c) Glut-1 staining of the junctional zone (a) and LZ (b) in Pdcd5+/+at E12.5, and of the junctional and labyrinth zones in Pdcd5–/–(c). (d) Transplacental passage of rhodamine 123 dye. One hour after tail vein injection of rhodamine 123, passive passage of the dye from the mother to the embryo was detected in Pdcd5+/+ and Pdcd5–/– embryos at E10.5
We next measured transplacental passage by injecting pregnant mice with the fluorescent dye rhodamine 123. As shown in Figure 4d, Pdcd5–/– embryos showed significantly less fluorescence than Pdcd5+/+ embryos. We also observed that Pdcd5–/– embryos were smaller than Pdcd5+/+ embryos.
To determine whether Pdcd5–/– placentas displayed abnormal proliferation or apoptosis, the placental specimens were stained with Ki-67. The number of Ki-67-positive cells was undetectable in the Pdcd5–/– JZ (Supplementary Figure S2b) compared with the Pdcd5+/+ JZ at E12.5 (Supplementary Figure S2a). The Ki-67 signal did not significantly differ between the Pdcd5–/– and Pdcd5+/+ LZs. We further assessed cell apoptosis by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay and cleaved caspase-3 staining. There were no significant differences between the Pdcd5–/– and Pdcd5+/+ placentas (Supplementary Figures S2c–e). Taken together, these results suggested that loss of Pdcd5 is associated with defects in placental development, accompanied by inhibition of cell proliferation.
Vegf, Vegfr-2 and Pecam-1 levels were decreased in the Pdcd5–/– LZ
The placenta is a vital organ facilitating the exchange of gases and nutrients between the fetus and the mother, and its physiological functions are closely related with vascular formation. Establishing a vascular network and blood circulation determine placental function, and protect the growth and development of the fetus. As the establishment of the vascular network in Pdcd5–/– placentas was defective (Figures 2 and 3), we attempted to analyze the regulatory mechanism of placental vasculogenesis.
Placental angiogenesis is regulated by vascular endothelial growth factor (Vegf) and its high-affinity receptor tyrosine kinases Vegfr. The results revealed that Vegf is highly expressed in maternal vascular endothelial cells in the Pdcd5+/+ LZ (Figure 5a). Vegfr-2, the primary Vegf receptor, is essential for normal endothelial proliferation and vascular formation. We found that the distribution of Vegfr-2 was consistent with that of Vegf in Pdcd5+/+ placentas (Figure 5b, left). On the contrary, Vegf and Vegfr-2-expressing cells were barely visible in the Pdcd5–/– LZ (Figures 5a and b).
PECAM-1 mediates endothelial integrity and other functions, including vascular permeability, regulation of bioavailability of nitric oxide and angiogenesis. Pecam-1 exhibited strong signaling in the Pdcd5+/+ LZ zone but not in the Pdcd5−/− placenta (Figure 5c). The Vegf, Vegfr-2 and Pecam-1 expression levels in fetal blood vessels were negative in the LZ of both Pdcd5–/– and Pdcd5+/+ placentas. These data suggest that Pdcd5 knockout strongly impaired endothelial integrity or function in mouse placental samples.
Expression of Pdcd5 in mouse placentas, embryonic livers and hearts
The embryonic lethality observed in Pdcd5–/– embryos indicated that Pdcd5 is indispensable for fetal development. We next analyzed the expression of Pdcd5 in WT placentas. As shown in Supplementary Figure S3, Pdcd5 was detected in both the cytoplasm and nucleus of the endothelial cells and cytotrophoblasts in the LZ (Supplementary Figure S3a). Pdcd5 was expressed in the nucleus of TGCs in the JZ at moderate levels (Supplementary Figure S3b). The lower immunoreactivity of Pdcd5 was detected in GlyTCs but not in SpTs (Supplementary Figure S3b). The DB cells expressed high or moderate levels of Pdcd5 in both the cytoplasm and the nucleus (Supplementary Figure S3c). The results of qRT-PCR revealed that the Pdcd5 mRNA levels were significantly lower in Pdcd5–/– placentas than in WT placentas, but completely absent in Pdcd5–/– embryos (Supplementary Figure S3d).
The levels of Pdcd5 mRNA were also detected by in-situ hybridization in E12.5 WT placenta. As shown in Supplementary Figures S3e–g, Pdcd5 mRNA was expressed at low or moderate levels in the LZs and JZs. In particular, the Pdcd5 mRNA signal was strong in the DB. We simultaneously analyzed Pdcd5 expression in WT embryonic livers and hearts at E12.5. Pdcd5 mRNA and protein levels were undetectable in embryonic livers (Supplementary Figures S4a and b, left panels), and low levels of Pdcd5 mRNA and protein were expressed in embryonic hearts (Supplementary Figures 4a and b, right panels). It should be pointed out that Pdcd5 mRNA levels were significantly higher in placentas than in embryonic livers and hearts.
Injured livers and hearts in Pdcd5 knockout mice
Embryonic development depends on successful placental maturation as well as cardiovascular maturation. Cardiovascular insufficiency is another major cause of embryonic lethality. Histological analysis of Pdcd5–/– embryos revealed defects in liver development. At E12.5, parenchymal hepatocytes became denser and formed hepatic lobules in the Pdcd5+/+ liver, while Pdcd5–/– livers displayed reduced cellularity and increased empty spaces, indicative of a relatively lower density of parenchymal hepatocytes (Figure 6a). At the same time, blood vessels including sinusoids were dilated, and numerous immature nucleated erythrocytes were observed in the Pdcd5–/– liver (Figure 6a, right), exhibiting hemorrhage. Cell apoptosis assay revealed strong signals of cleaved caspase-3 in Pdcd5–/– hepatocytes (Figure 6d).
Damaged livers and hearts in Pdcd5–/– embryos at E12.5. (a) The livers from Pdcd5+/+ or Pdcd5–/– embryos were analyzed by H&E staining. (b–c) The hearts from Pdcd5+/+ or Pdcd5–/– embryos were analyzed by H&E staining, magnified by 100 × (b) and 400 × (c). (d) The levels of cleaved caspase-3 protein in Pdcd5+/+ or Pdcd5–/– livers were detected by immunohistochemical analysis
Considering the core function of heart in circulation, morphogenetic defects in the heart can severely influence embryonic development. As shown in Figures 6b and c, the ventricular wall of the heart was thinner in Pdcd5–/– embryos than in their Pdcd5+/+ counterparts; however, the architecture in both the groups was normal. At a higher magnification, the number of myocardial cells was visibly lower in the ventricular wall of Pdcd5–/– embryos relative to that of Pdcd5+/+ embryos (Figure 6c). The atrial wall and papillary muscles of the Pdcd5–/– and Pdcd5+/+ embryos did not show obvious differences. Next, we investigated whether the thin ventricular wall in Pdcd5–/– heart was related to the increased apoptosis. Caspase-3 expression was not detected in both Pdcd5–/– and Pdcd5+/+ hearts (Supplementary Figure S5), indicating that the defect in the Pdcd5–/–heart might have no direct relationship with cell apoptosis. In view of the increased Pdcd5 expression in Pdcd5+/+ embryonic liver and heart at E14.5 (Supplementary Figure S4c), which occurred in Pdcd5−/− embryos around the time of death at E13.5 (Figures 1g and i), we considered that the major cause of embryonic lethality in Pdcd5-deficient mice may be placental failure. The abnormal phenomena observed in Pdcd5–/– embryonic livers and hearts may probably be indirect consequences of placental failure.
Pdcd5–/– MEF cells exhibited growth arrest and apoptosis
Based on the above findings, we further studied the biological activity of Pdcd5–/– mice embryonic fibroblasts (MEFs). At E10.5 or E11.5, Pdcd5–/– MEFs failed to adhere to the plate after separation 8 h up to 24 h, unlike Pdcd5+/+ MEFs (Supplementary Figures S6a and b). The size of Pdcd5–/– MEFs appeared smaller than Pdcd5+/+ MEFs. Results of the flow cytometry study further confirmed this phenotype (Supplementary Figures S6c). To verify this finding, we analyzed cell viability and growth kinetics using the CCK-8 and EdU (5-ethynyl-2′-deoxyuridine) incorporation assays. The results revealed that Pdcd5–/– MEFs were significantly less viable than Pdcd5+/+ MEFs (Figure 7a). The EdU-positive cells in the Pdcd5–/– MEFs were significantly lower than in the Pdcd5+/+ MEFs (Figures 7b and c), indicating that Pdcd5 knockdown inhibited cell proliferation.
Pdcd5–/– MEFs showed decreased proliferation, increased apoptosis and cell cycle arrest. (a) Cell viability was measured in Pdcd5+/+ and Pdcd5−/− MEF cells (E10.5) at indicated time using the CCK-8 assay. Data are means±S.D. of the results from three independent experiments (**P<0.01). (b) Pdcd5+/+ and Pdcd5−/− MEF cells at E10.5 were plated in glass slides and treated with EdU for 4 h, and then analyzed by immunofluorescence. Nuclei were stained with Hoechst 33342. Representative fluorescence microscopy images are shown. (c) Treated as (b), the ratio of EdU-positive cells to the total number of cells was counted in 10 visual fields and measured by the Student’s t-test. Data are means±S.D. of the results from three independent experiments (**P<0.01). (d) Pdcd5+/+ and Pdcd5−/− MEF cells at E10.5 were cultured for 12, 24 and 36 h, and then analyzed by Annexin V/propidium iodide (PI) staining and flow cytometry. (e) The cleaved caspase-3 levels in Pdcd5+/+ and Pdcd5−/− MEF cells at E10.5 were detected by western blot analysis. (f) Pdcd5+/+ and Pdcd5−/− MEF cells at E10.5 were cultured for 24 or 48 h, and then analyzed by PI staining and flow cytometry
We next determined cell apoptosis by measuring the phosphatidylserine externalization in MEFs. The results revealed a significantly higher number of apoptotic cells in Pdcd5–/– MEFs in a time-dependent manner (Figure 7d). This phenotype accompanied by caspase-3 activation (Figure 7e). At the same time, Pdcd5–/– MEFs showed a G0/G1 phase arrest at both 24 and 48 h (Figure 7f). Together, these data suggest that Pdcd5 knockdown could suppress cell growth, promote apoptosis and induce cell cycle arrest in MEFs.
Loss of Pdcd5 inhibited Hgf-mediated Pik3ca/Akt–Mtor signaling
To investigate the mechanism by which Pdcd5 knockout leads to embryonic lethality, we performed RT-PCR in embryos, placentas and MEFs to detect various signal pathway molecules. Among the detected genes, the Stat3, Tgf-β, Cadherin, Pten, β-catenin, Bmp4, Egfr and Hnf-4α transcript levels showed no obvious change in the mutant embryos, placentas and MEF cells (Figure 8a and Supplementary Figure S7a). Surprisingly, Hgf mRNA was distinctly decreased in Pdcd5–/– relative to that in the Pdcd5+/+ and Pdcd5–/+. The decreased transcription of Hgf in Pdcd5–/– embryos and placentas was further confirmed by quantitative RT-PCR (Figure 8b). The low levels of Hgf protein expressed in Pdcd5–/– placentas and embryos were confirmed by ELISA assay in tissue lysates (Figure 8c).
The Hgf–Pik3ca–Mtor signal pathway was inhibited after Pdcd5 knockout. (a–c) The Hgf mRNA and protein levels in different embryos and placentas at E11.5 were detected by RT-PCR (a), qRT-PCR (b) and ELISA (c). (d–f) Proteins from Pdcd5+/+ or Pdcd5–/– embryos at E11.5 were extracted and subjected to western blot analysis using the indicated antibodies
We then analyzed the changes in the signaling molecules downstream of Hgf to determine the signaling pathways that are affected by Pdcd5 knockdown. There was a significant decrease in Pik3ca (Y458) and Akt (S473) phosphorylation in the Pdcd5−/− embryos at E12.5 (Figure 8d). Simultaneously, the phosphorylation levels of Mtor at S2448, Rps6kb1 at T389 and Rps6 at S235/236 were also decreased in the Pdcd5–/– embryos (Figure 8e). Similar data were obtained in Pdcd5–/– MEFs (Supplementary Figure S7b). Additionally, loss of Pdcd5 had no significant influence on the phosphorylation of P38 Mapk, Erk 1/2, Lkb1 and Ampk (Figure 8f). Collectively, these results suggested that the embryonic lethality caused by Pdcd5 loss plays a role in the inhibition of the Hgf-regulated Pik3ca/Akt–Mtor signaling pathway.
Discussion
The results of the present study reveal the unique and unexpected role of Pdcd5 as a key regulator in the process of embryonic development. Genetic disruption of Pdcd5 resulted in impaired placental development, damaged placental angiogenesis and reduced nutrient supply to the fetus, consequently injuring the livers and hearts of embryos, leading to fetal growth retardation and death. The Pdcd5 levels were significantly higher in the placenta than in the embryonic liver and heart, suggesting that Pdcd5 may play a more important role in the placenta than in the fetus during embryonic development. Currently, it remains unknown whether the heart and liver defects were the primary reason for the observed embryonic lethal phenotype. Increase in the Pdcd5 expression occurred in Pdcd5+/+ embryonic livers and hearts at E14.5 (Supplementary Figure S4c), which lagged behind the increase in Pdcd5−/− embryos coinciding with embryonic death at E13.5 (Figures 1g and i). Based on these findings, we speculate that the abnormal phenomena in Pdcd5–/– embryonic liver and heart could be an indirect consequence of placental failure. In the present study, our findings demonstrate for the first time that Pdcd5 is a vital protein whose function cannot be compensated by other molecules in fetal development. Considering the pleiotropic functions ascribed to Pdcd5,21 it is likely that a multitude of factors contribute to embryonic lethality in mouse embryos lacking Pdcd5.
Several molecules that interact with Pdcd5, such as Vhl, Tip60, Hdac3 and Ck2, can cause embryonic lethality in the corresponding gene knockout mice. Histone acetyltransferase Tip60 and histone deacetylase Hdac3 interact with Pdcd5 to regulate Tp53 activation.3, 7 Ck2 mediates Pdcd5 phosphorylation and nuclear translocation. Vhl also participates in Pdcd5 location.21 Tip60 gene ablation causes early embryonic lethality at the eight-cell blastocyst stage (E3.5) and homozygous-null Tip60–/– mice do not survive up to the post-gastrulation stages of development.22 In another study, Hdac3–/– mice were reported to have died before E9.5 due to defects in gastrulation.23 Ck2-deficient embryos stopped development at the blastocyst stage and were resorbed at E7.5.24 Similarly, Vhl KO mice have been found to exhibit embryonic lethality as a result of abnormal placental development.25 Vhl-deficient mice died in utero from E10.5 to E12.5, following defective vasculogenesis and hemorrhage of the placental LZ. The Vegf levels in the labyrinth trophoblasts were greatly reduced in Vhl-deficient placentas relative to normal placentas;25 this is similar to the results obtained in Pdcd5–/– placentas. As a multitude of PDCD5-interacting molecules also trigger embryonic lethality when their expression levels are affected, Pdcd5 may function during embryonic development by regulating the above-mentioned molecules or in similar pathways.
The placenta is a highly vascularized tissue that develops during early gestation to facilitate the circulation of blood, oxygen, glucose and nutrients between the mother and the fetus. Placental failure was a major contributing factor to embryonic lethality. Trophoblast cells of the JZ are located at the trophoblast border of the uterus and can migrate into the DB and perform endocrine function. The LZ is the main site of contact between the fetal and maternal circulation, consisting of a highly branched fetal vascular network.19 Pdcd5 deletion affected placental vascularization (Figures 2, 3, 4, 5). Such alterations could impair embryonic growth and viability as the highly vascular LZ is the site of maternal–fetal oxygen and nutrient exchange.
TGCs are the first cell type to terminally differentiate during embryogenesis and are of vital importance for implantation and modulation of post-implantation placentation. TGCs mediate blastocyst attachment and invasion into the uterine epithelium, regulate decidualization of the uterus and anastomose with maternal blood spaces to form the transient yolk sac placenta. TGCs secrete a wide array of hormones and paracrine factors, including Vegf, Hgf and prolactin-related cytokines, to target the maternal physiological systems for proper maternal adaptations to pregnancy and the fetal–maternal interface to ensure vasculature remodeling.26, 27 TGCs also secrete urokinase-type plasminogen activator (uPA),28 which is a classical HGF activator to mediate pro-HGF cleavage and maturation.29 In Pdcd5 KO placentas, the number and size of TGCs had significantly decreased, resulting in a reduction in the Vegf and Hgf levels in placentas and embryos. Vegf-deficient mice underwent embryonic lethality before E8.5 due to the lack of vasculogenesis and blood island development.30 Hgf–/– mice embryos were found to exhibit reduced liver and placenta sizes and fewer hepatocytes and labyrinthine trophoblast cells relative to their wild-type counterparts. Moreover, the labyrinth region of the placenta was poorly developed, although the number of trophoblast cells in the JZ appeared normal.31, 32, 33 Based on these results, we can conclude that the decreased Vegf and Hgf levels in Pdcd5–/– mice may negatively regulate the expression and phosphorylation of Pik3ca/AKT–Mtor signaling, which occurred in the present study. This may play a contributory role in the defective vascularization and embryonic lethality.
Thus far, the roles of SpTs and GlyTCs in the JZ of mouse placenta remain unknown. It is thought that GlyTCs participate in glucose metabolism and utilize glucose as a source of energy.34 Glucose is one of the most important substances transferred from the maternal blood to the fetal circulation across the placenta, and it is a primary energy source for the fetus. Glucose transport to the fetus across the placenta takes place via glucose transporters, and the Glut-1 glucose transporter appears to be the rate-limiting step in transplacental transport.35 The number of SpTs and GlyTCs was obviously reduced in Pdcd5–/– placentas than in Pdcd5+/+ placentas, and the Glut-1 expression level was lower in the former than in the latter. In particular, the rhodamine 123 transport assay revealed that the placental nutrient transport function was decreased in Pdcd5–/– mice. Together with the insufficient placental vascularization, the lack of energy and nutrition in Pdcd5–/– mice was responsible for the abnormal embryogenesis, and ultimately death.
Many tumors have been shown to have reduced Pdcd5 levels, implicating its potential role as a tumor suppressor.21 However, heterozygous deletion of Pdcd5 in mice did not lead to the development of any tumors until 1 year of age (unpublished data). Perhaps the one remaining WT allele in heterozygous mice is sufficient to maintain the potential tumor suppressor function of Pdcd5. For many tumor suppressor genes, germline single-allele loss in combination with stochastic somatic loss has been shown to result in an increased incidence of tumors in certain organs. For example, total deletion of tumor suppressor gene Brac1 results in embryonic lethality, whereas mice heterozygous for Brac1 survived and did not develop tumors. Notably, introduction of a TP53-null allele significantly enhances mammary gland tumor formation in Brca1 conditional KO mice.36 Therefore, it is possible that another allele might function synergistically to enhance cancer development in Pdcd5 heterozygous mice. Future studies using Pdcd5 conditional knockout mice crossed with other mice tumor models will be necessary to identify if the embryonic lethality of Pdcd5 homozygous deletion can be overcome and to clarify the potential role of Pdcd5 as a tumor suppressor in vivo.
Materials and Methods
Generation of Pdcd5 knockout mice
Mice were maintained on a C57BL/6 genetic background. Mice harboring a floxed conditional knockout cassette of Pdcd5 were constructed by the Model Animal Research Center of Nanjing University (Nanjing, China). A FRT-Neo-FRT-LoxP cassette was inserted into intron 1 of the Pdcd5 gene and a LoxP site was inserted into intron 3. Mice carrying the floxed Pdcd5 allele (Pdcd5+/flox) were mated to mice expressing Cre recombinase under control of the Zp3 promoter (Zp3-Cre). The Cre recombinase can be expressed in the growing oocyte of Zp3-Cre transgenic mice prior to the completion of the first meiotic division.18 To generate heterozygous Pdcd5 knockout (Pdcd5+/−) mice, these Pdcd5+/flox:Zp3-Cre or Pdcd5flox/flox: Zp3-Cre mice were further mated with WT male mice. All mice were bred at the Experimental Animal Center, Peking University Health Sciences Center (Beijing, China) under a 12-h light/dark cycle. All mice were given free access to water and standard mouse chow. All protocols used within this study were approved by the Animal Care and Use Committee of the University.
Genotype analysis by PCR
Pdcd5 genotyping was performed by PCR using DNA isolated from the yolk sacs of embryos or from tails of postnatal mice. Tissues were soaked in 100 μl of 25 mM NaOH and 0.2 mM EDTA lysis buffer, heated for 30 min at 95 °C, and neutralized using 100 μl of 40 mM Tris-HCl (pH 5.5). The following primer sequences were used for Pdcd5 genotyping: P1, 5′-GGACTCCAGAGATGGTGCTCAG-3′; P2, 5′-TTGTCATGGTCATGGGAGCT-3′; and P3, 5′-TTTTCAGGCTTTACAAGTGC-3′. The following PCR cycling conditions were used: denaturation at 95 °C for 3 min; 35 cycles of amplification at 95 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s; and a final extension step at 72 °C for 5 min.
Antibodies and reagents
Rabbit anti-PDCD5 polyclonal antibodies used here have been described before.6 The following other antibodies were used in this study: anti-Ki-67 (ab16667), anti-HGF (ab83760), anti-GLUT-1 (ab115730), anti-VEGFA (ab52917) and anti-cleaved caspase-3 (ab13847) were purchased from Abcam (Cambridge, UK); and anti-PECAM-1 (SAB4502167), rhodamine 123 (R8004) and Hoechst 33342 (14533) were purchased from Sigma Aldrich (St. Louis, MO, USA). Signaling pathway-related antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA): anti-PIK3CA p85 (4292 s), anti-phospho- PIK3CA p85 (Try458, 4228 s), anti-AKT (9272 s), anti-phospho-AKT (Ser473, 4060 s), anti-Mtor (2983 s), anti-phospho-Mtor (Ser2448, 2971 s), anti-RPS6KB1 (2708 s), anti-phospho-RPS6KB1 (Thr389, 9234 s), anti-RPS6 (2217 s), anti-phospho-RPS6 (Ser235/236, 4858 s), anti-ERK (4695p), anti-phospho-ERK (Thr202/Tyr204, 4370 s), anti-AMPK (2532 s), anti-phospho-AMPK (Thr172, 2535 s), anti-LKB1 (3047 s), ani-phospho-LKB1 (Ser428, 3482 s), anti-p38 MAPK (9212p) and anti-phospho-p38 MAPK (Thr180/Tyr182, 4511p). Secondary antibodies included DyLight 800/DyLight 680-conjugated IgG against mouse (610-145-002/610-144-002, Rockland, Philadelphia, PA, USA) or rabbit (611-145-002/611-144-002, Rockland, Philadelphia, PA, USA) antibodies. RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40; P0013D) was purchased from Beyotime (Shanghai, China).
RT-PCR and quantitative real-time PCR (qRT-PCR)
Total RNA was prepared from embryos or placentas using TRIzol reagent (15596; Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using ReverAid First Strand cDNA Synthesis Kit (K1622; Invitrogen). mRNA expression was analyzed by RT-PCR or qRT-PCR and normalized to the expression of the Gapdh housekeeping gene. RT-PCR and qRT-PCR assays were performed in triplicate for each sample. Supplementary Table S2 lists the primers used for RT-PCR and qRT-PCR.
Western blot
Proteins from the embryos, placentas and MEFs were extracted using RIPA buffer containing phosphatase and protease inhibitor cocktail (04693116001/04906837001; Roche Diagnostics, Berlin, Germany). The cell lysates (80 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membrane (Whatman, Buckinghamshire, UK) and probed by the indicated antibodies. The protein bands were visualized using DyLight 800/DyLight 680-conjugated secondary antibodies, and an infrared fluorescence image was obtained using an Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Histological and immunohistochemical analysis
The embryos and placentas were fixed in formalin overnight and embedded in paraffin. For histopathological analysis, 4-μm sections were stained with hematoxylin and eosin (H&E) using standard procedures. A PAS staining kit (H0133; Harvey, Beijing, China) was used to stain glycogen-containing cells.
For immunohistochemical analysis, the embryo and placenta sections were dewaxed and rehydrated in xylol and a graded alcohol series containing decreasing concentrations of ethanol, followed by heat-induced antigen retrieval in citrate buffer. The sections were then treated by 3% H2O2 to quench endogenous peroxidase activity, and incubated with 5% goat serum. After incubation with the primary antibodies and biotinylated secondary antibody (PV-9000; Origen, Rockville, MD, USA), the color reaction was developed using 3,3′-diaminobenzidine (ZLI-9031; Origen).
Scanning electron microscopy
The placentas were fixed in formalin overnight and embedded in paraffin. For the scanning electron microscopy, 4-μm sections were placed on monocrystalline silicon piece, and dewaxed in xylol and ethanol. The sections were desalinated by repeated washing in deionized water and dehydrated in ethanol. The samples were coated with gold spray, and the sections were observed under a scanning electron microscope (S4800, JEOL, Tokyo, Japan).
Generation of MEF cells and cell culture
MEF cells were isolated from E10.5 or E11.5 mice embryos. Embryos were harvested, washed with phosphate-buffered saline, minced and trypsinized (0.25% trypsin) for 30 min at 25 °C. The cell suspensions were cultured in 10-cm dishes containing 10 ml of Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum and 1% penicillin/streptomycin in a CO2 incubator at 37 °C. The following studies of MEF cells were used in their second generation.
CCK-8 assay
Cell viability was determined with the CCK-8 reduction assay. MEF cells were seeded in a 96-well plate (3 × 103 cells/well) and incubated for 12, 36, 60 and 84 h. Then, 10 μl of CCK-8 solution was added to each well, and the cells were further incubated at 37 °C for 4 h. Absorbance was measured at 450 nm, and a reference was recorded at 630 nm with a microplate reader (Thermo, Waltham, MA, USA).
EdU incorporation assay
The EdU incorporation assay was performed using an EdU Imaging Kit (C10337; Invitrogen). MEF cells were seeded in the wells of a 24-well plate (1 × 104 cells per well) for 24 h, incubated with EdU at 37 °C for 4 h, fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100 and stained to observe EdU staining. The nuclei were stained by Hoechst 33342 for 5 min. Fluorescence signals were detected under a fluorescence microscope (BX53; Olympus, Miyazaki, Japan).
Apoptosis assay
Cell apoptosis was detected using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (Beijing Biosea Biotechnology Co., Ltd, Beijing, China) according to the manufacturer’s instructions. MEFs were first cultured in six-well plates (3 × 105 cells per well) for 12, 24 and 36 h, and collected and stained with Annexin V-FITC and propidium iodide (PI). The cells were then run through a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) to determine the percentage of apoptotic cells.
Tissue apoptosis was detected using an In Situ Cell Death Detection Kit (1168479591; Roche Diagnostics) by a TUNEL assay. Frozen placenta sections from both Pdcd5−/− and Pdcd5+/+ mice at E12.5 were treated and stained according to the manufacturer’s directions.
Cell cycle analysis
MEF cells were seeded in a six-well plate (3 × 105 per well) and cultured for 24 or 48 h. The cells were then fixed with 70% ethanol at 4 °C overnight, treated with 100 μg/ml RNase A for 30 min at 37 °C, stained using PI in 0.2% Triton X-100, and analyzed with a FACSCalibur flow cytometer (BD Biosciences).
Enzyme-linked immunosorbent assay (ELISA)
The Hgf in embyros and placentas were detected using a mouse ELISA Kit (ab100687; Abcam). The total protein was detected by a BCA protein assay kit (P0012; Beyotime). Data were presented as the ratio of Hgf protein level to the total protein level.
Statistical analysis
Data were presented as the means±S.D. Differences between groups were analyzed using the Student’s t-test for continuous variables. Statistical significance in this study was set at *P<0.05, **P<0.01 and ***P<0.001. All reported P-values are two-sided. All analyses were performed with GraphPad Prism 5 (GraphPad Software, Inc, La Jolla, CA USA).
References
Liu H, Wang Y, Zhang Y, Song Q, Di C, Chen G et al. TFAR19, a novel apoptosis-related gene cloned from human leukemia cell line TF-1, could enhance apoptosis of some tumor cells induced by growth factor withdrawal. Biochem Biophys Res Commun 1999; 254: 203–210.
Chen Y, Sun R, Han W, Zhang Y, Song Q, Di C et al. Nuclear translocation of PDCD5 (TFAR19): an early signal for apoptosis? FEBS Lett 2001; 509: 191–196.
Xu L, Chen Y, Song Q, Xu D, Wang Y, Ma D . PDCD5 interacts with Tip60 and functions as a cooperator in acetyltransferase activity and DNA damage-induced apoptosis. Neoplasia 2009; 11: 345–354.
Wang Y, Li X, Wang L, Ding P, Zhang Y, Han W et al. An alternative form of paraptosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci 2004; 117: 1525–1532.
Zhang S, Li G, Fu X, Qi Y, Li M, Lu G et al. PDCD5 protects against cardiac kremodeling by regulating autophagy and apoptosis. Biochem Biophys Res Commun 2015; 461: 321–328.
Xu L, Hu J, Zhao Y, Hu J, Xiao J, Wang Y et al. PDCD5 interacts with p53 and functions as a positive regulator in the p53 pathway. Apoptosis 2012; 17: 1235–1245.
Choi HK, Choi Y, Park ES, Park SY, Lee SH, Seo J et al. Programmed cell death 5 mediates HDAC3 decay to promote genotoxic stress response. Nat Commun 2015; 6: 7390.
Tracy CM, Gray AJ, Cuéllar J, Shaw TS, Howlett AC, Taylor RM et al. Programmed cell death protein 5 interacts with the cytosolic chaperonin containing tailless complex polypeptide 1 (CCT) to regulate beta-tubulin folding. J Biol Chem. 2014; 289: 4490–4502.
Murshed F, Farhana L, Dawson MI, Fontana JA . NF-kappaB p65 recruited SHP regulates PDCD5-mediated apoptosis in cancer cells. Apoptosis 2014; 19: 506–517.
Park SY, Choi HK, Choi Y, Kwak S, Choi KC, Yoon HG . Deubiquitinase OTUD5 mediates the sequential activation of PDCD5 and p53 in response to genotoxic stress. Cancer Lett 2015; 357: 419–427.
Park SY, Choi HK, Jo SH, Seo J, Han EJ, Choi KC et al. YAF2 promotes TP53-mediated genotoxic stress response via stabilization of PDCD5. Biochim Biophys Acta 2015; 1853: 1060–1072.
Cui X, Choi HK, Choi YS, Park SY, Sung GJ, Lee YH et al. DNAJB1 destabilizes PDCD5 to suppress p53-mediated apoptosis. Cancer Lett 2015; 357: 307–315.
Xiao J, Liu C, Li G, Peng S, Hu J, Qu L et al. PDCD5 negatively regulates autoimmunity by upregulating FOXP3(+) regulatory T cells and suppressing Th17 and Th1 responses. J Autoimmun 2013; 47: 34–44.
Xiao J, Li G, Hu J, Qu L, Ma D, Chen Y . Anti-inflammatory effects of recombinant human PDCD5 (rhPDCD5) in a rat collagen-induced model of arthritis. Inflammation 2015; 38: 70–78.
Xiao J, Liu W, Chen Y, Deng W . Recombinant human PDCD5 (rhPDCD5) protein is protective in a mouse model of multiple sclerosis. J Neuroinflammation 2015; 12: 117.
Li K, Zhou Z, Wang YO, Liu J, Zhao HB, Yang J et al. Pretreatment of mice with oligonucleotide prop5 protects them from influenza virus infections. Viruses 2014; 6: 573–581.
Jiang Z, Chen CH, Chen YY, Han JY, Riley J, Zhou CM . Autophagic effect of programmed cell death 5 (PDCD5) after focal cerebral ischemic reperfusion injury in rats. Neurosci Lett 2014; 566: 298–303.
Lewandoski M, Wassarman KM, Martin GR . Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol 1997; 7: 148–151.
Renaud SJ, Karim Rumi MA, Soares MJ . Review: Genetic manipulation of the rodent placenta. Placenta 2011; 32: S130–S135.
Illsley NP . Glucose transporters in the human place glucose transporters in the human placenta. Placenta 2000; 21: 14–22.
Li G, Ma D, Chen Y . Cellular functions of programmed cell death 5. Biochim Biophys Acta 2016; 1863: 572–580.
Hu Y, Fisher JB, Koprowski S, McAllister D, Kim MS, Lough J . Homozygous disruption of the Tip60 gene causes early embryonic lethality. Dev Dyn 2009; 238: 2912–2921.
Montgomery RL, Potthoff MJ, Haberland M, Qi X, Matsuzaki S, Humphries KM et al. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J Clin Invest 2008; 118: 3588–3597.
Buchou T, Vernet M, Blond O, Jensen HH, Pointu H, Olsen BB et al. Disruption of the regulatory beta subunit of protein kinase CK2 in mice leads to a cell-autonomous defect and early embryonic lethality. Mol Cell Biol 2003; 23: 908–915.
Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A et al. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA 1997; 94: 9102–9107.
Hu D, Cross JC . Development and function of trophoblast giant cells in the rodent placenta. Int J Dev Biol 2010; 54: 341–354.
Malassiné A, Frendo JL, Evain-Brion D . A comparison of placental development and endocrine functions between the human and mouse model. Hum Reprod Update 2003; 9: 531–539.
Martínez-Hernández MG, Baiza-Gutman LA, Castillo-Trápala A, Armant DR . Regulation of proteinases during mouse peri-implantation development: urokinase-type plasminogen activator expression and cross talk with matrix metalloproteinase 9. Reproduction 2011; 141: 227–239.
Naldini L, Tamagnone L, Vigna E, Sachs M, Hartmann G, Birchmeier W et al. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J 1992; 11: 4825–4833.
Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996; 380: 439–442.
Somerset DA, Afford SC, Strain AJ, Kilby MD . Fetal growth restriction and hepatocyte growth factor. Arch Dis Child Fetal Neonatal Ed 1997; 77: F244–F248.
Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 1995; 373: 702–705.
Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 1995; 37: 699–702.
Tesser RB, Scherholz PL, do Nascimento L, Katz SG . Trophoblast glycogen cells differentiate early in the mouse ectoplacental cone: putative role during placentation. Histochem Cell Biol 2010; 134: 83–92.
Baumann MU, Schneider H, Malek A, Palta V, Surbek DV, Sager R et al. Regulation of human trophoblast GLUT1 glucose transporter by insulin-like growth factor I (IGF-I). PLoS ONE 2014; 9: e106037.
Deng CX, Scott F . Role of the tumor suppressor gene Brca1 in genetic stability and mammary gland tumor formation. Oncogene 2000; 19: 1059–1064.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31370898, 91430101 and 81420108002), Beijing Natural Science Foundation (5142008) and the Specialized Research Fund for the Doctoral Program of Higher Education (20130001110015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by E Baehrecke
Supplementary Information
Materials and Methods
In situ hybridization. The embryos and placentas sections were dewaxed and rehydrated in xylol and a series of decreasing concentrations of ethanol, followed by 3% H2O2 for 10 min to quenched endogenous peroxidase activity, and pretreated with pepsin for 20 min. The sequence of Pdcd5 probe was 5’-AGTGGGAAGGTGTCAGAACAAGGTTTGATAGAAATACTTG-3’, labelled with digoxigenin at the 5' terminal. After pre-hybridized for 3 h, sections were hybridized overnight at 37°C with denatured cDNA probes (2 µg/ml) in hybridization solution. Subsequently, the sections were washed and the uncombined cDNA was removed. The sections were incubated with alkaline phosphatase-coupled sheep anti-digoxigenin antibody for 1 h at 37°C. The reaction was detected with a BlueMap kit (MK1031, Boster, Wuhan, China) and counterstained with nuclear fast red.
Transplacental passage of rhodamine 123. The pregnant mice from heterozygous matings were injected through tail vein at E10.5 with rhodamine 123 (1μg/g of body weight; R8004, Sigma-Aldrich, St. Louis, USA). The mice were sacrificed 1 h after the injection, and embryos were immediately analyzed with fluorescence microscope. The exposure time was 2 seconds for each embryo in same exciting light intensity. Embryos were then processed for genotyping.
Supplementary Figures

Figure 1
(JPG 304 KB)
Pdcd5+/+ and Pdcd5–/–- embryos at E12.5 were analyzed by hematoxylin and eosin (H&E) staining.

Figure 2
(JPG 898 KB) Decreased proliferation in Pdcd5–/– placenta junctional zone.
(a–b) Proliferation was detected by Ki-67 staining in Pdcd5+/+ (a) and Pdcd5–/– (b) junctional and labyrinth zones of the placentas at E12.5. (c) Apoptosis was detected by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay in both Pdcd5+/+ and Pdcd5–/– placentas at E12.5. (d-e) The levels of cleaved caspase-3 protein in Pdcd5+/+ (d) or Pdcd5–/– (e) junctional and labyrinth zones at E12.5 were detected by immunohistochemistry assay.

Figure 3
(JPG 1.18 MB) Expression of Pdcd5 in mouse placenta.
(a–c) Pdcd5 staining by immunohistochemistry in the Pdcd5+/+ labyrinth zone (a), junctional zone (b) and decidua basalis (c) of the placenta at E12.5. Trophoblasts are marked as trophoblast giant cells (asterisks), glycogen trophoblasts (triangles), and spongiotrophoblasts (hearts). (d) Relative Pdcd5 mRNA levels in E11.5 placentas and embryos were detected by qRT-PCR. Each group contained three samples and the expression was normalized to Gapdh (***p < 0.001). (e-g) The levels of Pdcd5 mRNA were detected in the WT placenta labyrinth zone (e), junctional zone (f) and decidua basalis (g) at E12.5 using in-situ hybridization.

Figure 4
(JPG 1.69 MB)
The expression of Pdcd5 in mouse embryonic liver and heart at E12.5 or E14.5 was detected by in-situ hybridization (a) and immunohistochemistry assay (b and c).

Figure 5
(JPG 511 KB)
Immunohistochemistry analysis of the expression of cleaved caspase-3 protein in Pdcd5+/+ and Pdcd5–/– heart at E12.5.

Figure 6
(JPG 439 KB) Survival ability of Pdcd5–/– MEF cells.
Representative microscopy images of Pdcd5+/+ and Pdcd5-/- MEF cells at E11.5 (a) and at E10.5 (b). (c and d) The morphology of Pdcd5+/+ and Pdcd5-/- MEF cells at E10.5 were analyzed by Flow cytometer.

Figure 7
(JPG 185 KB) The Hgf-Pi3kca-Mtor signal pathway was inhibited after Pdcd5 knockout in MEF cells.
(a) The levels of Hgf mRNA at E11.5 MEF were detected by RT-PCR. (b) Proteins from Pdcd5+/+ or Pdcd5-/- MEFs were extracted and subjected to western blot analysis using the indicated antibodies.
Supplementary Tables
Rights and permissions
Cell Death and Disease is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, G., Xu, C., Lin, X. et al. Deletion of Pdcd5 in mice led to the deficiency of placenta development and embryonic lethality. Cell Death Dis 8, e2811 (2017). https://doi.org/10.1038/cddis.2017.124
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2017.124
This article is cited by
-
Regulation of sexually dimorphic placental adaptation in LPS exposure-induced intrauterine growth restriction
Molecular Medicine (2023)
-
PDCD5 regulates iNKT cell terminal maturation and iNKT1 fate decision
Cellular & Molecular Immunology (2019)