Abstract
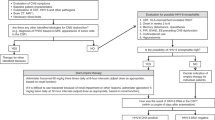
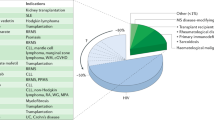
In this retrospective analysis using the Transplant Registry Unified Management Program, we identified 145 patients with human herpesvirus (HHV)-6 encephalitis among 6593 recipients. The cumulative incidences of HHV-6 encephalitis at 100 days after transplantation in all patients, recipients of bone marrow or PBSCs and recipients of cord blood were 2.3%, 1.6% and 5.0%, respectively. Risk factors identified in multivariate analysis were male sex, type of transplanted cells (relative risk in cord blood transplantation, 11.09, P<0.001; relative risk in transplantation from HLA-mismatched unrelated donor, 9.48, P<0.001; vs transplantation from HLA-matched related donor) and GvHD prophylaxis by calcineurin inhibitor alone. At 100 days after transplantation, the overall survival rate was 58.3% and 80.5% among patients with and without HHV-6 encephalitis, respectively (P<0.001). Neuropsychological sequelae remained in 57% of 121 evaluated patients. With both foscarnet and ganciclovir, full-dose therapy (foscarnet ⩾180 mg/kg, ganciclovir ⩾10 mg/kg) was associated with better response rate (foscarnet, 93% vs 74%, P=0.044; ganciclovir, 84% vs 58%, P=0.047). HHV-6 encephalitis is not rare not only in cord blood transplant recipients but also in recipients of HLA-mismatched unrelated donors. In this study, development of HHV-6 encephalitis was associated with a poor survival rate, and neurological sequelae remained in many patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Yamane A, Mori T, Suzuki S, Mihara A, Yamazaki R, Aisa Y et al. Risk factors for developing human herpesvirus 6 (HHV-6) reactivation after allogeneic hematopoietic stem cell transplantation and its association with central nervous system disorders. Biol Blood Marrow Transplant 2007; 13: 100–106.
Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M . Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis 2005; 40: 932–940.
Ogata M, Satou T, Kadota J, Saito N, Yoshida T, Okumura H et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis 2013; 57: 671–681.
Zerr DM, Ogata M In: Flamand L, Lautenschlager I, Krueger G, Ablashi D (eds). Human Herpesviruses HHV-6A, HHV-6B & HHV-7. Diagnosis and Clinical Management 3rd edn Elsevier: Oxford, UK, 2014 pp 217–234.
Ogata M, Fukuda T, Teshima T . Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation: what we do and do not know. Bone Marrow Transplant 2015; 50: 1030–1036.
Zerr DM . Human herpesvirus 6 (HHV-6) disease in the setting of transplantation. Curr Opin Infect Dis 2012; 25: 438–444.
Zerr DM . Human herpesvirus 6 and central nervous system disease in hematopoietic cell transplantation. J Clin Virol 2006; 37: 52–56.
Fujimaki K, Mori T, Kida A, Tanaka M, Kawai N, Matsushima T et al. Human herpesvirus 6 meningoencephalitis in allogeneic hematopoietic stem cell transplant recipients. Int J Hematol 2006; 84: 432–437.
Seeley WW, Marty FM, Holmes TM, Upchurch K, Soiffer RJ, Antin JH et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology 2007; 69: 156–165.
Muta T, Fukuda T, Harada M . Human herpesvirus-6 encephalitis in hematopoietic SCT recipients in Japan: a retrospective multicenter study. Bone Marrow Transplant 2009; 43: 583–585.
Mori Y, Miyamoto T, Nagafuji K, Kamezaki K, Yamamoto A, Saito N et al. High incidence of human herpes virus 6-associated encephalitis/myelitis following a second unrelated cord blood transplantation. Biol Blood Marrow Transplant 2010; 16: 1596–1602.
Hill JA, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant 2012; 18: 1638–1648.
Sakai R, Kanamori H, Motohashi K, Yamamoto W, Matsuura S, Fujita A et al. Long-term outcome of human herpesvirus-6 encephalitis after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17: 1389–1394.
Scheurer ME, Pritchett JC, Amirian ES, Zemke NR, Lusso P, Ljungman P . HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transplant 2013; 48: 574–580.
Dewhurst S . Human herpesvirus type 6 and human herpesvirus type 7 infections of the central nervous system. Herpes 2004; 11: 105A–111A.
Ljungman P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant 2008; 42: 227–240.
Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008; 47: 303–327.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol 2007; 86: 269–274.
Atsuta Y . Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol 2016; 103: 3–10.
Shintaku M, Kaneda D, Tada K, Katano H, Sata T . Human herpes virus 6 encephalomyelitis after bone marrow transplantation: report of an autopsy case. Neuropathology 2010; 30: 50–55.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Spitzer TR . Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27: 893–898.
Kishi Y, Kami M, Miyakoshi S, Kanda Y, Murashige N, Teshima T et al. Early immune reaction after reduced-intensity cord-blood transplantation for adult patients. Transplantation 2005; 80: 34–40.
Narimatsu H, Terakura S, Matsuo K, Oba T, Uchida T, Iida H et al. Short-term methotrexate could reduce early immune reactions and improve outcomes in umbilical cord blood transplantation for adults. Bone Marrow Transplant 2007; 39: 31–39.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Fine JP, Gray RJ . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Olson AL, Dahi PB, Zheng J, Devlin SM, Lubin M, Gonzales AM et al. Frequent human herpesvirus-6 viremia but low incidence of encephalitis in double-unit cord blood recipients transplanted without antithymocyte globulin. Biol Blood Marrow Transplant 2014; 20: 787–793.
Ogata M, Satou T, Kawano R, Takakura S, Goto K, Ikewaki J et al. Correlations of HHV-6 viral load and plasma IL-6 concentration with HHV-6 encephalitis in allogeneic stem cell transplant recipients. Bone Marrow Transplant 2010; 45: 129–136.
Uchida N, Wake A, Nakano N, Ishiwata K, Takagi S, Tsuji M et al. Mycophenolate and tacrolimus for graft-versus-host disease prophylaxis for elderly after cord blood transplantation: a matched pair comparison with tacrolimus alone. Transplantation 2011; 92: 366–371.
Zerr DM, Boeckh M, Delaney C, Martin PJ, Xie H, Adler AL et al. HHV-6 reactivation and associated sequelae after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1700–1708.
Manichanh C, Grenot P, Gautheret-Dejean A, Debre P, Huraux JM, Agut H . Susceptibility of human herpesvirus 6 to antiviral compounds by flow cytometry analysis. Cytometry 2000; 40: 135–140.
De Clercq E, Naesens L, De Bolle L, Schols D, Zhang Y, Neyts J . Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev Med Virol 2001; 11: 381–395.
Raffi F, Taburet AM, Ghaleh B, Huart A, Singlas E . Penetration of foscarnet into cerebrospinal fluid of AIDS patients. Antimicrob Agents Chemother 1993; 37: 1777–1780.
Morita D, Hirabayashi K, Katsuyama Y, Morokawa H, Motobayashi M, Kurata T et al. Viral load and ganciclovir (GCV) concentration in cerebrospinal fluid of patients successfully treated with GCV or valGCV for human herpesvirus 6 encephalitis/myelitis following umbilical cord blood transplantation. Transpl Infect Dis 2016; 18: 773–776.
Pellett PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol 2012; 22: 144–155.
Tanaka-Taya K, Sashihara J, Kurahashi H, Amo K, Miyagawa H, Kondo K et al. Human herpesvirus 6 (HHV-6) is transmitted from parent to child in an integrated form and characterization of cases with chromosomally integrated HHV-6 DNA. J Med Virol 2004; 73: 465–473.
Leong HN, Tuke PW, Tedder RS, Khanom AB, Eglin RP, Atkinson CE et al. The prevalence of chromosomally integrated human herpesvirus 6 genomes in the blood of UK blood donors. J Med Virol 2007; 79: 45–51.
Acknowledgements
We express our appreciation for the work of all of the physicians and data managers who contributed valuable data to the JSHCT and JDCHCT. We also thank members of the Transplant Registry Unified Management committees for their data management. This work was supported in part by the Practical Research Project for Allergic Diseases and Immunology from the Japan Agency for Medical Research and Development, AMED (to YA) and Research Program on Practical Research for Innovative Cancer Control from AMED (to TF).
Author contributions
MO designed the study, analyzed the data and wrote the manuscript. KO, TI and KT designed the study, analyzed the data and revised the manuscript. HK, TK, YU, TM, HH, HO, TE, TU and TM collected data and revised the manuscript. TI revised the manuscript and was responsible for data management for the JDCHCT. YA managed the registry database and revised the manuscript. TF designed the study, revised the manuscript and was responsible for the Complication Working Group.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Ogata, M., Oshima, K., Ikebe, T. et al. Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 52, 1563–1570 (2017). https://doi.org/10.1038/bmt.2017.175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.175
This article is cited by
-
Low urinary sodium-to-potassium ratio in the early phase following single-unit cord blood transplantation is a predictive factor for poor non-relapse mortality in adults
Scientific Reports (2024)
-
The preceding hyponatremia is a useful hallmark for the diagnosis of HHV-6 encephalitis after allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2023)
-
Coexistence of HLA and KIR ligand mismatches as a risk factor for viral infection early after cord blood transplantation
Bone Marrow Transplantation (2022)
-
Usefulness of the FilmArray Meningitis/Encephalitis Panel in diagnosis of central nervous system infection after allogeneic hematopoietic stem cell transplantation
Supportive Care in Cancer (2022)
-
HHV-6 associated diseases are one of the major factors on higher early CNS complications in CB recipients than in those of BM/PBSC
Bone Marrow Transplantation (2021)