Abstract
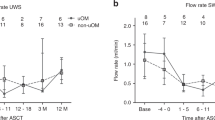
This randomized-controlled trial studied the efficacy of palifermin in a chemotherapy-only, high-dose Melphalan (HDM) transplant setting, to reduce oral mucositis (OM) and its sequelae measured by patient-reported outcomes (PRO) and medical resource use. Palifermin, relative to placebo was given either pre-/post-HDM or pre-HDM in patients with multiple myeloma (MM) undergoing auto-SCT at 39 European centers. Oral cavity assessment (WHO) and PRO questionnaires (oral mucositis daily questionnaire (OMDQ) and EQ 5D) were used in 281 patients (mean age 56, ±s.d.=8 years). 57 patients received placebo. One hundred and fifteen subjects were randomized to pre-/post-HDM receiving palifermin on 3 consecutive days before HDM and after auto-SCT and 109 patients were randomized to pre-HDM, receiving palifermin (60 μg/kg/day) i.v. for 3 consecutive days before HDM. There was no statistically significant difference in maximum OM severity. Severe OM occurred in 37% (placebo), 38% (pre-/post-HDM) and 24% (pre-HDM) of patients. No significant difference was observed with respect to PRO assessments or medical resource use, but more infections and fever during neutropenia were reported in pre-/post-HDM vs placebo (for example, 51 and 26%). To conclude, palifermin was unable to reduce OM or OM-related patient’s burden in MM transplant patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med 2004; 351: 2590–2598.
Blijlevens N, Schwenkglenks M, Bacon P, D'Addio A, Einsele H, Maertens J et al. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy—European Blood and Marrow Transplantation Mucositis Advisory Group. J Clin Oncol 2008; 26: 1519–1525.
Stiff PJ, Erder H, Bensinger WI, Emmanouilides C, Gentile T, Isitt J et al. Reliability and validity of a patient self-administered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT). Bone Marrow Transplant 2006; 37: 393–401.
Hochberg Y . A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988; 75 (4): 800–802.
Lehman EL . Nonparametrics. Holden-Day: San Francisco, 1975 pp 145.
Blazar BR, Weisdorf DJ, Defor T, Goldman A, Braun T, Silver S et al. Phase 1/2 randomized, placebo-control trial of palifermin to prevent graft-vs-host disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT). Blood 2006; 108: 3216–3222.
Spielberger R, Stiff P, Emmanouilides C . Efficacy of recombinant human keratinocyte growth factor (rHuKGF) in reducing mucositis in patients with hematologic malignancies undergoing autologous peripheral blood progenitor cell transplantation (auto-PBPCT) after radiation-based conditioning: results of a phase 2 trial (abstract no.25). Proc Am Soc Clin Oncol 2001; 20 (Pt 1): 7a.
Farrell CL, Bready JV, Rex KL, Chen JN, DiPalma CR, Whitcomb KL et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res 1998; 58: 933–939.
Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science 1994; 266: 819–822.
Sonis ST . The pathobiology of mucositis. Nat Rev Cancer 2004; 4: 277–284.
Potten CS, Booth D, Cragg NJ, Tudor GL, O'Shea JA, Booth C et al. Cell kinetic studies in the murine ventral tongue epithelium: mucositis induced by radiation and its protection by pretreatment with keratinocyte growth factor (KGF). Cell Prolif 2002; 35 (Suppl 1): 32–47.
Zia-Amirhosseini P, Salfi M, Leese P, Yates W, Danilenko DM, Ring B et al. Pharmacokinetics, pharmacodynamics, and safety assessment of palifermin (rHuKGF) in healthy volunteers. Clin Pharmacol Ther 2006; 79: 558–569.
Vadhan-Raj S, Trent J, Patel S, Zhou X, Johnson MM, Araujo D et al. Single-dose palifermin prevents severe oral mucositis during multicycle chemotherapy in patients with cancer: a randomized trial. Ann Intern Med 2010; 153: 358–367.
Lerman MA, Treister NS . Generalized white appearance of the oral mucosa. Hyperkeratosis secondary to palifermin. J Am Dent Assoc 2010; 141: 867–869.
Schroeder T, Zohren F, Saure C, Kobbe G, Palifermin HaasR . A recombinant human keratinocyte growth factor, triggers reactivation of anogenital human papillomavirus infection in a HIV-positive patient with diffuse large cell B-cell non-Hodgkin lymphoma. Bone Marrow Transplant 2009; 44: 823–824.
Kobbe G, Bruns I, Schroeder T, Czibere A, Warnecke J, Hieronimus N et al. A 3-day short course of palifermin before HDT reduces toxicity and need for supportive care after autologous blood stem-cell transplantation in patients with multiple myeloma. Ann Oncol 2010; 21: 1898–1904.
Elting LS, Shih YC, Stiff PJ, Bensinger W, Cantor SB, Cooksley C et al. Economic impact of palifermin on the costs of hospitalization for autologous hematopoietic stem-cell transplant: analysis of phase 3 trial results. Biol Blood Marrow Transplant 2007; 13: 806–813.
Langner S, Staber P, Schub N, Gramatzki M, Grothe W, Behre G et al. Palifermin reduces incidence and severity of oral mucositis in allogeneic stem-cell transplant recipients. Bone Marrow Transplant 2008; 42: 275–279.
Goldberg JD, Zheng J, Castro-Malaspina H, Jakubowski AA, Heller G, van den Brink MR et al. Palifermin is efficacious in recipients of TBI-based but not chemotherapy-based allogeneic hematopoietic stem cell transplants. Bone Marrow Transplant 2012; 48: 99–104.
Peterson DE, Bensadoun RJ, Roila F . Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol 2011; 22 (Suppl 6): vi78–vi84.
Grazziutti ML, Dong L, Miceli MH, Krishna SG, Kiwan E, Syed N et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant 2006; 38: 501–506.
Acknowledgements
Study investigators were as follows (listed alphabetically): Emanuele Angelucci, Cagliari Italy; Lotfi Benboubker, Tours France; Nicole Blijlevens, Nijmegen the Netherlands; Paul Browne, Dublin Ireland; Jean-Henri Bourhis, Villejuif France; Donald Bunjes, Ulm Germany; Angelo Michele Carella, San Martino Italy; Charles Crawley, Cambridge UK; Ilse Christiansen, Aalborg Denmark; Johannes Clausen, Innsbruck Austria; Peter Dreger, Heidelberg Germany; Hermann Einsele, Wurzburg Germany; Gordon Cook, Leeds UK; Mark Cook, Birmingham UK; Thierry Facon, Lille France; Michael Fillitz, Vienna Austria; Laurent Garderet, Villejuif France; Alois Gratwohl, Basel Switzerland; Jean Luc Harrousseau, Nantes France; Pasquale Iacopino, AO Melacrino Morelli Italy; Ladislav Jebavy, Hradec Kralove Czech Republic; Jan-Erik Johansson, Gothenburg Sweden; Hedvig Kasparu, Linz Austria; Nicolas Ketterer, Lausanne Switzerland; Joachim Kienast, Muenster Germany; Guido Kobbe, Dusseldorf Germany; Gergely Krivan, Budapest Hungary; Giorgio Lambertenghi Deliliers, Milan Italy; Roberto M. Lemoli, Bologna Italy; Werner Linkesch, Graz Austria; Hajna Losonczy, Pecs Hungary; Johan Maertens, Leuven Belgium; Dieter Lutz, Linz Austria; Giovanna Meloni Roma Italy; Tamas Masszi, Budapest Hungary; Gareth Morgan, London UK; Dietger Niederwieser, Leipzig Germany; Robert Pytlik, Prague Check Republic; Werner Rabitsch, Wien Austria; Amin Rahemtulla, London UK; Jean-Francois Rossi, Montpellier France; Nigel Russell, Nottingham UK; Tapani Ruutu, Helsinki Finland; Giuseppe Saglio, Turin Italy; Ann Sonet, Yvoir Belgium; Arpad Szomor, Pécsi Hungary Jan Van Droogenbroeck, Brugge Belgium; Pierre Zachee, Antwerpen Belgium. This study was supported by Swedish Orphan Biovitrum AB. Author contribution: Nicole Blijlevens, Maarten de Château, Dietger Niederwieser designed research, performed research, analyzed data and wrote the paper. Gergely Krivan, Werner Rabitsch, Arpad Szomor, Robert Pytik, Hans E Johnsen, Hermann Einsele, Tapani Ruutu performed research and wrote the paper. Theo de Witte wrote the paper. Agneta Lissmats designed research, analyzed data and wrote the paper.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
Maarten de Château employment by Swedish Orphan Biovitrum AB, medical program director. Agneta Lissmats employment by Swedish Orphan Biovitrum AB, biostatistician. The remaing authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Blijlevens, N., de Château, M., Krivan, G. et al. In a high-dose melphalan setting, palifermin compared with placebo had no effect on oral mucositis or related patient’s burden. Bone Marrow Transplant 48, 966–971 (2013). https://doi.org/10.1038/bmt.2012.257
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.257
Keywords
This article is cited by
-
Methylene blue for intractable pain from oral mucositis related to cancer treatment: a randomized phase 2 clinical trial
BMC Medicine (2022)
-
Mitigating acute chemotherapy-associated adverse events in patients with cancer
Nature Reviews Clinical Oncology (2022)
-
Palifermin, administered for three doses only, reduces mucositis in patients undergoing HSCT and receiving chemoradiotherapy conditioning
Bone Marrow Transplantation (2022)
-
Translational model of melphalan-induced gut toxicity reveals drug-host-microbe interactions that drive tissue injury and fever
Cancer Chemotherapy and Pharmacology (2021)
-
Anakinra: efficacy in the management of fever during neutropenia and mucositis in autologous stem cell transplantation (AFFECT-2)—study protocol for a multicenter randomized double-blind placebo-controlled trial
Trials (2020)