Abstract
Oral mucositis (OM) is a frequent complication of stem cell transplantation-associated toxicity in haematological malignancies, contributing to mortality. Therapy still remains mainly supportive. We assessed risk factors in retrospective analysis of 192 autologous peripheral stem cell transplantation patients with lymphoma and multiple myeloma (MM), respectively. Futhermore, we examined the hormone levels both in serum and saliva during transplantation in 7 postmenopausal female patients with lymphoma compared to healthy controls using electrochemiluminescence immunoassay (ECLIA). Multivariable analysis revealed neutrophil engraftment (p < 0.001; p = 0.021) and female sex (p = 0.023; p = 0.038) as independent predictive factors in the combined patient group and in the lymphoma group, and neutrophil engraftment (p = 0.008) in the MM group. Of the 85 female participants 19 were pre- and 66 postmenopausal. Fifteen of the pre-, and 49 of the postmenopausal women developed ulcerative mucositis (p = 0.769), more often with lymphoma than MM (p = 0.009). Serum estrogen decreased significantly both in postmenopausal controls and transplantated patients compared to premenopausals, with no difference in saliva. Serum progesterone level was significantly (p = 0.026) elevated at day + 7 of transplantation, while salivary progesterone increased at day + 7 and + 14. Our results indicate a predominantly negative effect of female sex hormones on oral immunity with role in the aetiopathogenesis of OM.
Similar content being viewed by others
Introduction
There is a rise in incidence of malignant haematological diseases. Autologous and allogeneic haematopoietic stem cell transplantations (HSCT) are used with increasing frequency and efficiency as a therapy1. High-dose intensive cytostatic treatment and in some cases total body irradiation (TBI) is employed as part of the transplantation process (myeloablative conditioning therapy). Oral mucositis (OM) is a common and severe complication, graded 0–4 according to the World Health Organization (WHO)2. Anticancer treatment-related mucosal damage affects not only the oral cavity but also the entire gastrointestinal tract (GI), therefore it is more appropriate to use the umbrella term of mucosal barrier injury (MBI). OM causing severe pain and extensive ulcers decreases quality of life and due to damage the protective mucosal barrier results in the dissemination of oral pathogens, leading to sepsis, which is often fatal in protracted neutropenia. Prolonged hospital stay increases the risk of nosocomial infections with high mortality and strain on the health care system3. OM has several patient and anti-tumoural therapy related risk factors, such as nutritional deficiency, poor dental hygiene, previous exposure to prolonged immunosuppressive therapy and genetic variations affecting the pharmacodynamics of cytostatics4. Excretion of chemotherapeutic drugs in the saliva is locally cytotoxic2,5. Decreased salivary flow rate and altered saliva composition may enhance OM risk and severity6. Despite strenuous efforts in research and therapy, OM treatment remains mainly supportive and palliative with basic oral care, pain control, feeding and prevention of infections7. However, there are no predictive markers regarding the severity of OM in HSCT. Our goal was to assess and identify novel risk factors which could predict the severity of OM in haematological patients undergoing autologous peripheral stem cell transplantation (APSCT).
Materials and methods
Retrospective study population and design
In our study we conducted a restrospective analysis of 192 patients over a period of 4 years who had required and undergone APSCT due to malignant haematological disorder in the Haematopoietic Transplantation Centre of the Clinical Centre of the University of Debrecen, Hungary. The study was approved by the Regional Istitutional Research Ethics Committee, Clinical Center, University of Debrecen (Ethical licence: DE RKEB/IKEB 4948-2018). Diagnoses were obtained from the institutional electronic clinical database eMedSolution (T-Systems Inc. Budapest, Hungary) in accordance with the ethical approval. Written informed consents for inclusion, intraoral examination, blood and saliva sampling and research participation were obtained from all subjects and data were anonymised. No organs/tissues were procured from prisoners. All methods were carried out in accordance with relevant guidelines and regulations. The study was conducted in accordance with the Declaration of Helsinki. Eight patients were excluded due to non-compliance mainly related to disease severity.
Two large patient groups were created, lymphoma (Hodgkin (HL) and non-Hodgkin’s lymphoma (NHL)) and multiple myeloma (MM). Regarding stage at disease onset all patients were early and advanced, respectively, while regarding stage prior to the transplantation we established two groups: complete remission (CR) and very good partial remission (VGPR) were merged as a single group, whereas partial remission (PR) represented a separate group. Response categories were determined in accordance with the International Myeloma Working group (IMWG) uniform response criteria8. VGPR was defined as follows: serum and urine M-protein detectable by immunofixation but not on electrophoresis, or: 90% reduction in serum M-protein plus reduction in 24 h urinary M-protein by > 90% or to < 100 mg/24 h. In partial remission more than 50% reduction of serum M-protein can be observed, and reduction in 24 h urinary M-protein by ≥ 90% or to < 200 mg/24 h, and 50% reduction in the size of any plasmacytomas present at baseline. In Hodgkin lymphoma we categorised the conditioning treatments applied during the transplantation into four groups (1. BEAM, 2. R-BEAM, 3. R-BEAM-Adcetris, and 4. other). In NHL, in group 1 R-BEAM and in group 2 Z-BEAM conditioning regimen was used, whereas group 3 represented cases with any other conditioning regimen. In MM conditioning was administration of 140 mg/m2 melphalan in 12 patients and 200 mg/m2 in the rest of the patients. Treatment regimens according to the European Society for Blood and Marrow Transplantation (EBMT) recommendation9,10 are summarized in Supplementary Tables S1–S4. None of the patients received cryotherapy. After transplantation all patients received G-CSF with the following antimicrobial prohylaxis: (1) antibacterial prophylaxis: Leflokin (levofloxacin) 500 mg, (2) antifungal prophylaxis: Mycosyst (fluconazole) 100 mg and (3) antiviral prophylaxis: Herpesin (acyclovir) 400 mg. OM was classified according to the World Health Organization (WHO) guidelines (Grade 0–4) and the most severe appearence defined the stage in the individual patient (0 none; 1 soreness ± erythema; 2 erythema, ulcers, and patient can swallow solid food; 3 ulcers with extensive erythema and patient cannot swallow solid food; 4 mucositis to the extent that alimentation is not possible)2.
For the statistical analysis there were two separate groups, (1) non-ulcerative (OM0-1) and (2) ulcerative (OM2-4) mucositis11. Each participant had a check-up for focal dental infections and removal of infection foci prior to the transplantation. During transplantation, patients rinsed their mouths with 0.2% chlorhexidine-digluconate (CHX) solution and performed atraumatic mechanical plaque control using a special dento-brush (DenTips MDS096502, Medline Industries. Inc., Mundelein, IL, USA) after every meal during cytopenia. The time required for neutrophil engraftment was calculated as number of days with < 0.5 Giga (109) per Litre (G/L) absolute neutrophil count (ANC), and for thrombocyte engraftment as number of days with < 20 G/L thrombocyte (THR) count.
We analysed the relationship between oral mucositis developed during transplantation and the following continuous variables: age at time of transplantation, time elapsed between diagnosis and transplantation (DG-TX time/month), amount of stem cells administered (106/body mass kg), stem cell viability (%), number of viable cells (106/body mass kg) and mononuclear cells (MNC) (108/body weight kg), engraftment time (ANC < 0.5 G/L, THR < 20 G/L-days) and LDH (U/L). Of the categorical variables we analysed the relationship between oral mucositis and sex; stage of the disease at diagnosis (early versus advanced) and prior to transplantation (PR, VGPR, CR); the type of conditioning applied; outcome (dead or alive); infectious complications in the early post-transplantation stage (positive haemoculture); and correlation with disease subtype, where applicable. VGPR and CR, the stages immediately preceding transplantation, were conflated according to standard practice. Neutrophil engraftment is defined by peripheral absolute neutrophil count of ≥ 0.5 G/L, whereas thrombocyte engraftment by ≥ 20 G/L thombocytes, in three consecutive days12. Thus in our study we characterised the time of engraftment with the ˂ 0.5 G/L ANC and ˂ 20 G/L THR days, respectively.
Based on their presumed hormonal status, the 85 female patients of the study were separated into premenopausal (≤ 50 years) (n = 19) and postmenopausal (≥ 51 years) (n = 66) groups, respectively, according to published criteria13. All premenopausal APSCT women were on hormone replacement therapy (HRT) (5–10 mg norethisterone-acetate on cycle days 3–27, prior to and after the day of the transplantation, as long as cytopenia persisted in order to reduce the risk of severe haemorrhagic complications).
Prospective study population and design
Estradiol (E2) and progesterone (P4) levels in serum and saliva were determined in respective healthy controls (7 pre- and 7 postmenopausal women) and in 7 postmenopausal patients with NHL undergoing APSCT, at four stages of transplantation (day − 7/− 3, 0, + 7, + 14). Sampling was performed in the Haematopoietic Transplantation Centre of the Clinical Centre of the University of Debrecen, Hungary in an 8-week period ending December 15th, 2018.
In regarding this measurement study design was aligned with STROBE recommendations and using sample size calculator Sampsize (epiGenesys, Sheffield, UK) this part of the study was a pilot. Average age was 66.71 ± 5.85 years in the postmenopausal (A), 25.43 ± 1.62 years in the premenopausal (C) control group while 54.42 ± 12.29 years in the transplantated group. Three patients were in complete morphological remission (CMR) and 4 in partial remission (PR) prior to transplantation.
Collection of serum samples
Blood and saliva samplings were performed at the same timepoint on specified days of the peritransplantation period (on the day of hospital admission (day − 3/− 7), day of transplantation (day 0), days + 7 and + 14). Blood samples were collected using a clot activator containing serum tubes (BD, Franklin Lakes, NJ, USA), centrifuged at 1,200 × g for 30 min and the serum fractions were stored at − 70 °C until further processing.
Collection of unstimulated whole saliva (UWS) samples
Sample collection was based on the protocols described previously14,15. In brief, following oral cavity rinse with 25 ml of physiological saline solution (B. Braun Melsungen AG, Melsungen, Germany) for 30 s, saliva was collected for 5 min in 15 ml disposable, pre-disinfected tubes (Sigma-Aldrich, St. Louis, MO, USA). Participants adapted to the condition for 5 min. Taking into account the diurnal variation of saliva constituents, samplings were done every morning at the same time, 1 h after eating, drinking or toothbrushing. Patients in the Haematopoietic Stem Cell Transplantation Centre’s steril rooms used a steril disposable oral swab (DenTips MDS096502, Medline Industries.Inc., Mundelein, IL, USA) impregnated with physiological saline solution to maintain oral hygiene during the period of cytopenia. Protease Inhibitor (Sigma-Aldrich, St. Louis, MO, USA) was added to the collected saliva samples and after aliquoting into 1.5 ml Eppendorf tubes, samples were stored at − 70 °C until processing.
Detection of salivary and blood sample E2 and P4 levels
Saliva and serum samples stored at − 70 °C were thawed at room temperature and centrifuged at 4 °C for 10 min at 3,000 rpm. Serum (500 µl) and diluted saliva supernatant (150 µl in 450 µl Hanks’ Balanced Salt solution (Sigma-Aldrich, St. Louis, MO, USA) were filtered through 70 µm EASYstrainer cell sieve (Greiner Bio-One, Frickenhausen, Germany). Hormone levels were determined using electrochemiluminescence immunoassay (ECLIA) (Roche, Basel, Switzerland).
Statistical analysis
Statistical analysis was performed using IBM SPSS22 software (IBM, Armonk, NY, USA). Kolmogorov–Smirnov test was used to investigate the normal distribution of data. In case of normal distribution, we compared the two groups using independent sample t-test in the continuous variables, whereas in non-normal distribution we applied Mann–Whitney and Wilcoxon tests. For distribution of categorical variables Chi-square test and Fischer exact test were used. Overall survival (OS) was calculated from the time of diagnosis to the last follow-up visit or death. Survival data were analyzed using the Kaplan–Meier method with log-rank test. Odds Ratios (OR) were obtained using binary logistic regression models. p < 0.05 was considered significant.
Results
Retrospective analysis of primary outcome of patients who underwent APSCT
The reason for HSCT was lymphoma (NHL-HL) in 85 patients and MM in 107 patients. Mean age of the 107 male patients was 54.87 ± 11.64 years (17–72 years) and of the 85 female patients was 56.13 ± 11.38 years (24–71 years). There was no significant difference between NHL-HL and MM groups regarding age and sex. The OM grade varied from grade 1 (n = 4), 2 (n = 9), 3 (n = 7) to 4 (n = 4) in HL; from grade 1 (n = 35); 2 (n = 37), 3 (n = 20) to 4 (n = 8) in MM; from grade 1 (n = 14), 2 (n = 19), 3(n = 18) to 4 (n = 7) in NHL. Considering all patients irrespective of underlying disease (n = 192), 10 did not have OM (grade 0), 53 developed grade 1, 65 had grade 2, 45 had grade 3, and 19 had grade 4 OM. In the combined lymphoma group (NHL-HL) 18 patients showed grade 1, 28 grade 2, 25 grade 3, and 11 grade 4 OM (Supplementary Fig. S1).
In the all patient group continuous variables DG-TX time (pre-treatment time) (p = 0.022), neutrophil engraftment (p < 0.001), thrombocyte engraftment (p < 0.001) and categorical variable female sex (p = 0.033) showed a significant correlation with more severe OM. There was a positive non-significant association between OM2-4 and higher LDH value (p = 0.07) and haemoculture positivity (p = 0.092). In the lymphoma group the continuous variables neutrophil engraftment (p = 0.005) and thrombocyte engraftment (p = 0.005) and the categorical variable female sex (p = 0.008) showed a significant correlation with OM2-4. In the myeloma group, the neutrophil engraftment (p = 0.011) and immunoglobulin G (IgG) (p = 0.049) subgroup of MM showed a significant correlation with OM2-4. The other examined variables showed no correlation with the development of oral mucositis occurring during APSCT (Table 1).
Univariable analysis revealed in the all patient group in terms of OM2-4 the following prognostic factors: neutrophil engraftment (OR 1.461, 95% CI 1.225–1.744, p < 0.001), thrombocyte engraftment (OR 1.178, 95% CI 1.078–1.288, p < 0.001), female sex (OR 1.969, 95% CI 1.052–3.687, p = 0.034) and lymphoma group (OR 1.969, 95% CI 1.052–3.678, p = 0.034). Increased LDH values (OR 1.004, 95% CI 1–1.009, p = 0.067) and cases with positive haemoculture (OR 1.901, 95% CI 0.894–1.041, p = 0.095) may represent higher risks in the development of more severe OM. In the lymphoma subgroup, in addition to neutrophil engraftment (OR 1.584, 95% CI 1.134–2.214, p = 0.007), thrombocyte engraftment (OR 1.279, 95% CI 1.043–1.57, p = 0.018) and female sex (OR 5.294, 95% CI 1.418–19.762, p = 0.013), absence of B symptoms (OR 7.000, 95% CI 1.085–45.16, p = 0.041) turned out to be prognostic factors of OM2-4. Hodgkin and non-Hodgkin’s lymphoma subgroups were combined due to the small number of cases in the HL group. Absence of B symptoms by itself did not appear as a prognostic factor among patients with NHL. In the myeloma group, neutrophil engraftment (OR 1.39, 95% CI 1.09–1.773, p = 0.008) and thrombocyte engraftment (OR 1.128, 95% CI 1.009–1.262, p = 0.034) were prognostic factors while the IgG subgroup (OR 2.766, 95% CI 0.984–7.773, p = 0.054) suggests a higher risk for the development of OM.
Multivariable analysis revealed that in the all patient group and in the lymphoma group neutrophil engraftment (OR 1.492, 95% CI 1.228–1.813, p < 0.001; OR 1.476, 95% CI 1.061–2.052, p = 0.021) and female sex (OR 2.301, 95% CI 1.124–4.714, p = 0.023; OR 4.190, 95% CI 1.081–16.240; p = 0.038) could be considered independent predictive factors (Classification ratio: 67.7%, Nagelkerke coefficient: 0.172); (Classification ratio: 78.8%, Nagelkerke coefficient: 0.236) in the development of OM2-4. In the myeloma group neutrophil engraftment (OR 1.39, 95% CI 1.09–1.773, p = 0.008) (Classification ratio: 62.6%, Nagelkerke coefficient: 0.105) appeared as an independent prognostic factor (Table 2).
Time to neutrophil engraftment (7.774 ± 2.10 days) was significantly (p < 0.001) longer in the lymphoma group than in the MM (5.80 ± 2.07 days). At the same time, neutrophil engraftment was the strongest predictive factor in the NHL group (OR 1.598, 95% CI 1.101–2.321, p = 0.014); (thrombocyte engraftment: OR 1.239, 95% CI 1.004–1.529, p = 0.046; female sex: OR 5.320, 95% CI 1.077–26.276, p = 0.040) (Fig. 1).
Risk factors and independent predictive factors of oral mucositis. ANC absolute neutrophil count, HL Hodgkin lymphoma, IgG immunoglobulin G, LDH lactate-dehydrogenase, NHL Non-Hodgkin’s lymphoma, THR thrombocyte count. Flowchart summary of statistical tests’ results. Colour codes/Letter markings: Red: independent prognostic factors revealed by multivariable analysis Black: prognostic factors revealed by multivariable analysis Green: higher risk in terms of ulcerative mucositis by univariable analysis.
Secondary outcomes of retrospective analysis of women who underwent APSCT
As next, since female sex has been shown to have a major role in the development of OM2-4, we conducted further analysis.
The 85 female patients were classified into two groups based on their hormonal status13. Patients ≤ 50 years were grouped as premenopausal and those ≥ 51 years as postmenopausal, as we mentioned earlier. Based on this classificiation 19 women were premenopausal and 66 postmenopausal. Of the 19, 15 (78.95%), and of the 66, 49 (74.24%) patients developed ulcerative mucositis (OM2-4). There was no significant difference between the two groups (p = 0.771).
We did our calculations for the total patient group and the individual patient group, respectively, and got similar results. In the lymphoma subgroup 16 (88.89%) out of 18 premenopausal women, while 14 (93.33%) out of 15 postmenopausal women developed severe OM. The difference was not significant here, either (p = 1). In the myeloma group, 33 out of 49 postmenopausal female patients developed ulcerative OM while the corresponding ratio in the premenopausal group was 1 out of 3 (33.33%). No significant difference was found here, either (p = 0.114).
However, ulcerative mucositis was significantly (p = 0.009) more frequent in the lymphoma group than in the MM group (30 of the 33 patients with lymphoma-90.9%, while 34 out of the 53 patients with MM-65.38%, p = 0.009).
Overall survival with and without ulcerative mucositis
Correlation was assessed between average post-transplantational survival time and ulcerative mucositis. OS was 5.17 months shorter in the combined patient group (HL, NHL, MM) if ulcerative mucositis developed (OM2-4: 35.34 months (31.83–38.85); OM0-1: 40.51 months (26.42–44.61) (p = 0.101) (Fig. 2).
Determination of serum and salivary E2 and P4 levels
A physiological decrease was observed in serum E2 level in the postmenopausal control group (A) compared to the premenopausal (C) (p = 0.004), while there was no significant difference in salivary E2 level (p = 0.069). Both in serum and saliva, P4 levels were significantly decreased in the postmenopausal controls compared to the premenopausal group (p = 0.017, p = 0.004).
Serum P4 was more elevated in the transplantated patients compared to the postmenopausal controls at all four stages of transplantation, at day + 7 significantly (p = 0.026). Salivary P4 was higher, although not significantly at days + 7 and + 14 compared to the two other stages of APSCT and to controls (≥ 51), respectively. Although decrease in salivary P4 level was significant in the postmenopausal controls compared to the premenopausals, a tendency for increase was observed in postmenopausal APSCT patients at day + 7 and day + 14 compared not only to the postmenopausal controls, but also to the premenopausals (Fig. 3).
Saliva and serum levels of estrogen (E2) and progesterone (P4) in the pre- and postmenopausal control groups and during APSCT (autologous peripheral stem cell transplantation). (A) Serum and salivary E2 levels in the control groups. Serum E2 decreased significantly (p = 0.004) in the postmenopausal group compared to the premenopausal group. There was no significant difference (p = 0.069) in salivary E2 levels. (B) Serum and salivary P4 levels in the control groups. Both in serum and saliva P4 levels were significantly decreased in the postmenopausals compared to the premenopausals (p = 0.017, p = 0.004). (C) Serum P4 levels in the postmenopausal control group compared to the transplantated patients. Significant difference was observed at day + 7 (0.026). (D) Salivary P4 levels in the postmenopausal control group and in the transplantated group. A tendency for increase was observed at days + 7 and + 14. (E) Salivary P4 levels in the premenopausal control group and in the transplantated patients. Physiological significant decreased was observed in remission (day − 3/ − 7) and day 0, and a tendency for elevation at days + 7 and + 14 compared to the premenopausal controls. On the X axis C is for premenopausal control group and A is for postmenopausal control group. Values are expressed as sample means; error bars represent the estimates of standard deviations calculated from three parallel measurements (*p < 0.05, **p < 0.01, ***p < 0.001). Statistical analysis: Kolmogorov–Smirnov test, Mann–Whitney test, Wilcoxon test.
Serum E2 decreased significantly (p = 0.004) in the patient group compared to the premenopausal controls, while there was no significant difference between E2 serum and salivary hormone levels of postmenopausal controls and patients with NHL undergoing APSCT in the postmenopause. In summary, we didn’t find any significant changes of E2 in relation to NHL and/or due to the APSCT in the patient group.
Unstimulated whole saliva (UWS) in controls and in patients during APSCT
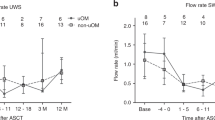
There was no significant difference (p = 0.628) in UWS flow rate between the pre- and postmenopausal control groups. During APSCT, significant decrease was observed at day 0, day + 7 and day + 14 in UWS flow rate between the pre- and postmenopausal control groups (p = 0.004, p = 0.004, p = 0.004); (p = 0.048, p = 0.030, p = 0.018), and between the day of admission of APSCT (day − 3/− 7) (p = 0.043, p = 0.043, p = 0.043), respectively (Fig. 4). There was a significant positive correlation (p = 0.008, r = 0.928) between serum E2 level and UWS flow rate in the premenopausal group.
Salivary flow rate of unstimulated whole saliva (UWS) in controls and in patients during autologous peripheral stem cell transplantation (APSCT). On the X axis C is for premenopausal control group and A is for postmenopausal control group. Values are expressed as sample means; error bars represent the estimates of standard deviations calculated from three parallel measurements (*p < 0.05, **p < 0.01, ***p < 0.001). Statistical analysis: Kolmogorov–Smirnov test, Mann–Whitney test and Wilcoxon test.
Discussion
OM is associated with increased mortality in haematological malignancies. Numerous studies have attempted to prevent it or reduce its severity, with limited success7. There is a new wave in OM studies with focus on risk factors implicated in other diseases of the oral cavity including cancer. Our results indicate that neutrophil engraftment is an independent predictive factor of OM both in lymphoma and MM whereas female sex predisposes to ulcerative OM in lymphoma only.
Serum LDH levels
Pre-transplantation serum LDH levels are a good reflection of the remaining tumour mass, thus its prognostic role is important. Huang et al. examined the prognostic value of pre-radiotherapy serum LDH levels in patients with non-metastatic oropharyngeal carcinoma (OPC)16. Their results showed that increased LDH levels in HPV+ OPC patients were an independent prognostic factor in terms of survival. The results of our own study suggest that with marginally increased pre-transplantational serum LDH levels a higher prevalence of ulcerative oral mucositis can be predicted, which supports its negative prognostic role.
Neutrophil engraftment and oral mucositis
Neutrophils in the first lines of defense play a crucial role in protection againts infections and promote wound healing17. Platelets contribute to tissue remodelling after injury and inflammation18. Timely insertion and proliferation of stem cells in neutrophil and thrombocyte engraftments are essential in reducing risk and severity of OM. Earlier studies have reported at length on the relationship between neutrophil engraftment and OM, and the positive correlation has also been confirmed between the duration of mucositis and neutrophil engraftment19. Reduced as opposed to standard dose MTX in allogeneic HSCT patients resulted in shorter neutrophil engraftment time and faster recovery from mucositis19. The anti-inflammatory, antioxidant and wound-healing promoting effects of erythropoietin containing mouthwash in patients who underwent APSCT, reducing severity and incidence of OM without significant effect on blood neutrophil and thrombocyte engraftment12, further supports the role of local factors in the aetiology and therapy of OM. Several studies have unanimously concluded that oral engraftment is an earlier sign of adherence and recovery of stem cells than blood engraftment. Furthermore, it is a more sensitive indicator of oral mucositis and reflects the status of local immunity20,21,22.
Female sex
The predictive role of the female sex regarding OM in APSCT has been confirmed by earlier studies23,24, however, no in-depth analysis like our study has been reported. The dose of chemotherapy applied over a larger body surface area (BSA) per body mass in women may explain the sex difference25. Furthermore, women have increased risk of cytotoxicity and oral mucositis possibly partially due to hormone-related post-translational modifications26. We confirmed that APSCT-related OM was more frequent and severe in women than in men. Severe ulcerative OM (OM2-4) was more frequent in lymphoma than in myeloma, pontentially due to the longer pre-treatment time. There was no significant difference in incidence of ulcerative OM between pre- and postmenopauls. To explain this, we conducted hormonal analysis in the prospective part of our study. In the postmenopause E2 levels were physiologically decreased (Fig. 3A). In premenopausal patients, hormone levels were affected by progestin and norethisterone-acetate administered before and during transplantation to reduce the risk of haemorrhagic complications. However, we observed significantly increased serum progesterone levels at day + 7 of APSCT (which is the deepest point of cytopenia and the most severe grade of OM) also in postmenopausal patients with lymphoma (Fig. 3C), who also had increased incidence and severity of OM in our retrospective analysis. Therefore, we assume that the hormonal changes identified in our study (increased progesterone in postmenopausal patients in particular) do exert a strong effect on OM development. It is generally known that sex hormones have direct and indirect effects on the physiology of the oral cavity. In future studies, the effects of hormonal changes on the oral epithelium, periodontium, oral microbiome, the composition of the saliva and function of the immune system could be correlated with tissue-specific localization, quantity and functional status of sex hormone receptors.
Oral epithelium
The adverse effect of decreased serum estrogen on the mucosa is well known: the oral epithelium becomes thin and atrophic, accompanied by a deterioration of mucosal integrity and immunity27, whereas estrogen supplementation and HRT improves mucosal integrity28,29,30. The oral mucosa also responds to progesterone31, high levels being detrimental, especially in immuno-compromised patients32,33,34. There were elevated serum and salivary progesterone levels in our patients during APSCT (Fig. 3C,D,E).
Periodontium and oral microbiome
The increased sensitivity of periodontal tissues to sex hormones has been widely investigated. Estrogen increases epithelial keratinization, stimulates proliferation of fibroblasts and epithelial basal cells in the gingiva27,35. In postmenopausal women the prevalence of periodontitis is significantly higher compared with premenopausals36. Periodontal pathogens such as Porphyromonas gingivalis, Tannerella forsythia, Prevotella intermedia play a role in the development and progression of periodontitis, while HRT decreases the amount of this pathogen flora37,38. In our patients with APSCT-related cytopenia the cycle was suspended with norethisterone-acetate. This progestin decreases keratinization, favours colonisation of Gram-negative bacteria (e.g. Bacteroides, Prevotella sp.). Progesterone also enhances inflammatory response to local irritative factors thereby increasing the risk and severity of OM35. These findings support the notion that the predictive role of female sex in the development of OM during APSCT is a consequence of the shifted hormonal balance.
During HSCT, patients with chronic gingivitis or periodontitis have higher incidence of bacteriaemia and ulcerative mucositis39. In the preengraftment stage there is often anaerob blood stream infection (ABI) with Fusobacterium and Porphyromonas species of oral and upper GI tract origin, whereas during postengraftment Bacteroides and Clostridium species of lower GI tract origin colonize40. Changes in microbial diversity occure rapidly in patients with ulcerative oral mucositis during APSCT41. However, we did not find significant correlation between evidence of infection by haemoculture and ulcerative oral mucositis (OM2-4) in our retrospective study. Futher research is recommended with wider range of methods, such as swab culture and polymerase chain reaction (PCR).
In conclusion, hormonal changes (particularly decreased E2 and increased P4 levels) have a negative effect on the periodontium and favour the proliferation of periodonthopathogens, which play an increased role in the development of bacteriaemia and ulcerative OM during HSCT.
Salivary glands and saliva
Saliva has a fundamental role in the homeostasis of the oral cavity, lubrication of the mucosa, and protection against microorganisms and oxidative stress. Changes in its organic and anorganic components act as a sensitive mirror of the body’s defence mechanisms. Its diagnostic importance is outstanding and comparable with the serum. Steroid hormone levels in the saliva usually correlate with the active hormone levels in the serum42,43. Our salivary P4 results correlated well with serum P4 levels both in healthy controls and in the transplantated patients (Fig. 3), whereas it did not reach the level of significance regarding salivary E2. Salivary secretion and changes in its components are under significant sex hormone regulation and estrogen and progesterone receptors are present in the salivary glands31,44. Most studies report decreased salivary flow rate in postmenopause noting the positive effect of HRT45,46, while others enhance the decrease of stimulated whole saliva only47,48. We found no significant difference in UWS flow rate between the pre-and postmenopausal controls, and a positive correlation between serum E2 level and UWS flow rate in the premenopausals (Fig. 4). Several studies have demonstrated decreased salivary flow rate during APSCT under the influence of conditioning treatment6, in concert with our results (Fig. 4).
There is impairment of salivary antimicrobial defence and mucosal integrity during APSCT6,49. Thus, we can conclude that postmenopausal hormonal changes increase the risk of OM that occurs during APSCT.
Oral immunity
Although the interactions involved are not yet completely understood, the interrelationship between production of sex hormones and the functioning of the immune system has been confirmed in many studies35. This is also supported by increased incidence of autoimmune diseases among women35. Estrogen has a stimulating effect on the immune system, as opposed to progesterone and androgens50. E2 regulates glycosylation of IgG and its role in immunological processes51. Also, salivary secretory immunoglobulin A (sIgA) levels change parallel to serum E2 levels52 and altered N-glycosylation of salivary sIgA and serum IgA is a possible biomarker in oral mucositis15. Importantly, high-dose progesterone attenuates the antibacterial activity of neutrophils53, whereas decreased estrogen aggravates dento-alveolar infections54 and impairs leukocyte chemotaxis35. These observations further underline the importance of sex hormones in OM and their relevance to APSCT. Their role in PMN chemotaxis may be crucial in ulcerative OM development and support our finding that neutrophil engraftment is an independent prognostic factor of OM.
Conclusion
Hormonal changes in women play a fundamental role in the aetiopathogenesis of OM during APSCT. These changes may exert their complex effect directly or indirectly on components of saliva and on oral epithelium, microbiome and immunity. Decreased estrogen levels adversely affect several aspects of oral homeostasis, and elevated progesterone levels both in serum and saliva may play a role in the weakening of the mucosal barriers not only in the pre- but also in postmenopause in particular during APSCT. Monitoring serum progesterone levels in women undergoing APSCT may be a suitable tool in the assesment of mucosal immunity, function and risk of severe OM.
Data availability
The data sets used for analysis for this study are available from the corresponding author upon reasonable request.
Abbreviations
- ABI:
-
Anaerobic bloodstream infection
- ANC:
-
Absolute neutrophil count
- APSCT:
-
Autologous peripheral stem cell transplantation
- BEAM:
-
BCNU, etoposide, cytosine arabinoside, melphalan
- BMI:
-
Body mass index
- CHX:
-
Chlorhexidine digluconate
- CI:
-
Confidence interval
- CMR:
-
Complete morphological remission
- CR:
-
Complete remission
- DG:
-
Diagnosis
- E2:
-
17-beta-estradiol
- GI:
-
Gastrointestinal tract
- G-CSF:
-
Granulocyte-colony stimulating factor
- G/L:
-
Giga per Litre
- GVHD:
-
Graft-versus-host disease
- HL:
-
Hodgkin lymphoma
- HSCT:
-
Haematopoietic stem cell transplantation
- HRT:
-
Hormone replacement therapy
- IgA:
-
Immunoglobulin A
- IgG:
-
Immunoglobulin G
- IMWG:
-
International Myeloma Working Group
- LDH:
-
Lactate-dehydrogenase
- MTX:
-
Methotrexate
- MBI:
-
Mucosal barrier injury
- MM:
-
Multiple myeloma
- NHL:
-
Non-Hodgkin lymphoma
- OM:
-
Oral mucositis
- OM0-1:
-
Non-ulcerative mucositis
- OM2-4:
-
Ulcerative mucositis
- OR:
-
Odds ratios
- OPC:
-
Oropharyngeal carcinoma
- OS:
-
Overall survival
- P4:
-
Progesterone
- PCR:
-
Polymerase chain reaction
- PR:
-
Partial remission
- PMN:
-
Polymorphonuclear leukocyte
- R-BEAM:
-
Rituximab-BCNU, etoposide, cytosine arabinoside, melphalan
- sIgA:
-
Secretory immunoglobulin A
- TBI:
-
Total body irradiation
- THR:
-
Thrombocyte
- TX:
-
Transplantation
- UWS:
-
Unstimulated whole saliva
- VGPR:
-
Very good partial remission
- WHO:
-
World Health Organization
- Z-BEAM:
-
Zevalin-BCNU, etoposide, cytosine arabinoside, melphalan
References
Kanate, A. S. et al. Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 26, 1247–1256 (2020).
Niscola, P. et al. Mucositis in patients with hematologic malignancies: an overview. Haematologica 92, 222–231 (2007).
Blijlevens, N. M. A., Logan, R. M. & Netea, M. G. Mucositis: from febrile neutropenia to febrile mucositis. J. Antimicrob. Chemother. 63, i36–i40 (2009).
Salavaggione, O. E., Wang, L., Wiepert, M., Yee, V. C. & Weinshilboum, R. M. Thiopurine S-methyltransferase pharmacogenetics: variant allele functional and comparative genomics. Pharmacogenet. Genom. 15, 801–815 (2005).
Sonis, S. T. et al. Perspectives on cancer therapy-induced mucosal injury. Cancer 100, 1995–2025 (2004).
van Leeuwen, S. et al. Early salivary changes in multiple myeloma patients undergoing autologous HSCT. Oral Dis. 24, 972–982 (2018).
Jensen, S. B. & Peterson, D. E. Oral mucosal injury caused by cancer therapies: current management and new frontiers in research. J. Oral Pathol. Med. 43, 81–90 (2014).
Durie, B. et al. International uniform response criteria for multiple myeloma. Leukemia 20, 1467–1473 (2006).
Storti, F. et al. Melphalan 140 mg/m2 or 200 mg/m2 for autologous transplantation in myeloma: results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT Chronic Malignancies Working Party. Haematologica 103, 514–521 (2018).
Gyurkocza, B. & Sandmaier, B. M. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 124, 344–353 (2014).
Chaudhry, H. M. et al. The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: a systematic review. Biol. Blood Marrow. Transplant. 22, 605–616 (2016).
Hosseinjani, H. et al. The efficacy of erythropoietin mouthwash in prevention of oral mucositis in patients undergoing autologous hematopoietic SCT: a double-blind, randomized, placebocontrolled trial. Hematol. Oncol. 35, 106–112 (2017).
McKinlay, S. M., Brambilla, D. J. & Posner, J. G. The normal menopause transition. Am. J. Hum. Biol. 4, 37–46 (1992).
Meszaros, B. et al. N-glycomic analysis of Z(IgA1) partitioned serum and salivary immunoglobulin A by capillary electrophoresis. Curr. Mol. Med. https://doi.org/10.2174/1566524020666200413114151 (2020).
Gebri, E. et al. N-glycosylation alteration of serum and salivary immunoglobulin A is a possible biomarker in oral mucositis. J. Clin. Med. 9, 1747 (2020).
Huang, S. H. et al. Independent adverse prognosis of elevated serum LDH in human papillomavirus-related oropharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 96, S83 (2016).
Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 371, 531–539 (2018).
Kasirer-Friede, A. et al. The platelet response to tissue injury. Front. Med. 5, 317 (2018).
Matsukawa, T. et al. Reduced-dose methotrexate in combination with tacrolimus was associated with rapid engraftment and recovery from oral mucositis without affecting the incidence of GVHD. Int. J. Hematol. 104, 117–124 (2016).
Akpek, G., Knight, R. D. & Wright, D. G. Use of oral mucosal neutrophil counts to detect the onset and resolution of profound neutropenia following high-dose myelosuppressive chemotherapy. Am. J. Hematol. 72, 13–19 (2003).
Cheretakis, C., Dror, Y. & Glogauer, M. A noninvasive oral rinse assay to monitor engraftment, neutrophil tissue delivery and susceptibility to infection following HSCT in pediatric patients. Bone Marrow Transplant. 36, 227–232 (2005).
Pink, R. et al. Salivary neutrophils level as an indicator of bone marrow engraftment. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 153, 263–269 (2009).
Vokurka, S. et al. Higher incidence of chemotherapy induced oral mucositis in females: a supplement of multivariate analysis to a randomized multicentre study. Support. Care Cancer 14, 974–976 (2006).
Valeh, M. et al. Factors affecting the incidence and severity of oral mucositis following hematopoietic stem cell transplantation. Int. J. Hematol. Stem Cell Res. 12, 142–153 (2018).
Blijlevens, N. et al. Prospective oral mucositis audit: oral mucositis in patients receiving high-dose melphalan or BEAM conditioning chemotherapy—European Blood and Marrow Transplantation Mucositis Advisory Group. J. Clin. Oncol. 26, 1519–1525 (2008).
Huang, R. S., Kistner, E. O., Bleibel, W. K., Shukla, S. J. & Dolan, M. E. Effect of population and gender on chemotherapeutic agent-induced cytotoxicity. Mol. Cancer Ther. 6, 31–36 (2007).
Alzoubi, E. E. Oral manifestations of menopause. J. Dent. Health Oral Disord. Ther. 7, 306–309 (2017).
Litwack, D., Kennedy, J. E. & Zander, H. A. Response of oral epithelia to ovariectomy and estrogen replacement. J. Periodontal Res. 5, 263–268 (1970).
Tarkkila, L., Furuholm, J., Tiitinen, A. & Meurman, J. H. Saliva in perimenopausal and early postmenopausal women. A 2-year follow-up study. Clin. Oral Investig. 16, 767–773 (2012).
Croley, T. E. & Miers, C. Epithelial changes in the oral mucosa resulting from a variation in hormone stimulus. J. Oral Med. 33, 86–89 (1978).
Välimaa, H. et al. Estrogen receptor-β is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 180, 55–62 (2004).
Solomon, M., Itsekson, A. M. & Lev-Sagie, A. Autoimmune progesterone dermatitis. Curr. Dermatol. Rep. 2, 258–263 (2013).
Grunnet, K. M., Powell, K. S., Miller, I. A. & Davis, L. S. Autoimmune progesterone dermatitis manifesting as mucosal erythema multiforme in the setting of HIV infection. JAAD Case Rep. 3, 22–24 (2017).
Minicucci, E. M. et al. Oral stomatitis induced by endogenous progesterone: case report. Gynaecol. Endocrinol. 25, 543–545 (2009).
Mariotti, A. Sex steroid hormones and cell dynamics in the periodontium. Crit. Rev. Oral Biol. Med. 5, 27–53 (1994).
Haas, A. N., Rösing, C. K., Oppermann, R. V., Albandar, J. M. & Susin, C. Association among menopause, hormone replacement therapy, and periodontal attachment loss in Southern Brazilian Women. J. Periodontol. 80, 1380–1387 (2009).
Brennan, R. M., Genco, R. J., Hovey, K. M., Trevisan, M. & Wactawski-Wende, J. Clinical attachment loss, systemic bone density, and subgingival calculus in postmenopausal women. J. Periodontol. 78, 2104–2111 (2007).
Tarkkila, L., Kari, K., Furuholm, J., Tiitinen, A. & Meurman, J. H. Periodontal disease-associated micro-organisms in peri-menopausal and post-menopausal women using or not using hormone replacement therapy. A two-year follow-up study. BMC Oral Health 10, 10 (2010).
Raber-Durlacher, J. E. et al. Periodontal status and bacteremia with oral viridans streptococci and coagulase negative staphylococci in allogeneic hematopoietic stem cell transplantation recipients: a prospective observational study. Support. Care Cancer 21, 1621–1627 (2013).
Okinaka, K. et al. Characteristics of anaerobic bloodstream infections after allogeneic hematopoietic stem cell transplantation. J. Hematop. Cell Transplant. 6, 52–59 (2017).
Laheij, A. M. G. A. et al. Microbial changes in relation to oral mucositis in autologous hematopoietic stem cell transplantation recipients. Sci. Rep. 9, 1–11 (2019).
Greabu, M. et al. Saliva—a diagnostic window to the body, both in health and in disease. J. Med. Life 2, 124–132 (2009).
Tivis, L. J., Richardson, M. D., Peddi, E. & Arjmandi, B. Saliva versus serum estradiol: Implications for research studies using postmenopausal women. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 29, 727–732 (2005).
Shick, P. C., Riordan, G. P. & Foss, R. D. Estrogen and progesterone receptors in salivary gland adenoid cystic carcinoma. Oral Surg. Oral Med Oral Pathol. Oral Radiol. Endod. 80, 440–444 (1995).
Mahesh, D. R. et al. Evaluation of salivary flow rate, PH and buffer in pre, post & post menopausal women on HRT. J. Clin. Diagn. Res. 8, 233–236 (2014).
Nagler, R. M. & Hershkovich, O. Relationships between age, drugs, oral sensorial complaints and salivary profile. Arch. Oral Biol. 50, 7–16 (2005).
Fenoll-Palomares, C. et al. Débito basal, pH y capacidad tampón de la secreción salivar en sujetos sanos. Rev. Esp. Enferm. Dig. 96, 773–783 (2004).
Minicucci, E. M., Pires, R. B. C., Vieira, R. A., Miot, H. A. & Sposto, M. R. Assessing the impact of menopause on salivary flow and xerostomia. Aust. Dent. J. 58, 230–234 (2013).
Avivi, I. et al. Oral integrity and salivary profile in myeloma patients undergoing high-dose therapy followed by autologous SCT. Bone Marrow Transplant. 43, 801–806 (2009).
McMurray, R. W. Estrogen, prolactin, and autoimmunity: Actions and interactions. Int. Immunopharmacol. 1, 995–1008 (2001).
Ercan, A. et al. Estrogens regulate glycosylation of IgG in women and men. JCI Insight 2, e89703 (2017).
Gómez, E. et al. Hormonal regulation of the secretory IgA (sIgA) system: estradiol- and progesterone-induced changes in sIgA in parotid saliva along the menstrual cycle. Am. J. Reprod. Immunol. 29, 219–223 (1993).
Roth, J. A., Kaeberle, M. L. & Hsu, W. H. Effect of estradiol and progesterone on lymphocyte and neutrophil functions in steers. Infect. Immun. 35, 997–1002 (1982).
Youssef, H. & Stashenko, P. Interleukin-1 and estrogen protect against disseminating dentoalveolar infections. Int. J. Oral Sci. 9, 16–23 (2017).
Acknowledgements
This work was supported by the Faculty of Dentistry University of Debrecen Research Fund GINOP-2.3.2.15-2016-0001; the Hungarian Brain Research Program (2017-1.2.1-NKP-2017-00002), GINOP-2.3.2-15-2016-00043; SZTE ÁOK-KKA NO. 5S 567 (A202); Hungarian Scientific Research Fund (OTKA-NKFIH-SNN 132999); DE ÁOK Research Fund (TH). We are grateful to Professor Csaba Hegedűs, Ágnes Kinga Bágyi and István Varga for providing research facilities. The help of Drs Réka Ágoston and Helga Sipos in sample collection, Miss Katalin Hodosi in statistical analysis, and Miss Julianna Hortobágyi in proofreading is thankfully appreciated. We are thankful to all clinicians and health professionals who contributed to the diagnosis, treatment and care of the patients enrolled in this study.
Author information
Authors and Affiliations
Contributions
Conception and design of the study by E.G., T.H. and A.K. Primary draft and revisions by E.G. and T.H. Patient assessment by E.G., A.K. Hormone analysis by E.G., F.T. Statistical tests by E.G. All authors have read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gebri, E., Kiss, A., Tóth, F. et al. Female sex as an independent prognostic factor in the development of oral mucositis during autologous peripheral stem cell transplantation. Sci Rep 10, 15898 (2020). https://doi.org/10.1038/s41598-020-72592-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-72592-5
This article is cited by
-
Sex-dependent differences in the genomic profile of lingual sensory neurons in naïve and tongue-tumor bearing mice
Scientific Reports (2023)
-
Prospective longitudinal analysis of clinical and immunological risk factors associated with oral and gastrointestinal mucositis following autologous stem cell transplant in adults
Supportive Care in Cancer (2023)
-
The effects of Ankaferd hemostat on preventing oral mucositis in colorectal cancer patients receiving chemotherapy
Supportive Care in Cancer (2023)
-
Is the Sex Difference a Clue to the Pathomechanism of Dry Eye Disease? Watch out for the NGF-TrkA-Piezo2 Signaling Axis and the Piezo2 Channelopathy
Journal of Molecular Neuroscience (2022)
-
Confounding factors in the assessment of oral mucositis in head and neck cancer
Supportive Care in Cancer (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.