Abstract
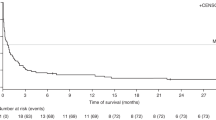
The largest study on post-allogeneic hematopoietic cell transplant lymphoproliferative disorder (PTLD) epidemiology showed a cumulative incidence of 1.7% in patients receiving antithymocyte globulin (ATG). We had noted an apparently higher incidence in our transplant recipients whose conditioning included ATG. Therefore, we formally determined the incidence of PTLD through chart review. We also evaluated whether counts of EBV-specific T lymphocytes measured by cytokine flow cytometry could identify patients at risk of developing PTLD. Among 307 allogeneic transplant recipients, 25 (8.1%) developed PTLD. This was biopsy proven in 11 patients, and was fatal in seven patients. Patient age, EBV serostatus, donor type/match or GVHD did not influence PTLD risk significantly. Median onset of PTLD was 55 (range, 28–770) days post transplant. Day 28 EBV-specific T lymphocyte counts were not significantly different in 11 patients who developed PTLD and 31 non-PTLD patients matched for published risk factors for PTLD. In summary, when using conditioning with thymoglobulin 4.5 mg/kg, the incidence of PTLD is relatively high and cannot be predicted by day 28 cytokine flow cytometry-determined EBV-specific T lymphocyte counts. Thus, in this scenario PTLD prevention may be warranted, for example, using EBV DNAemia monitoring with preemptive therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Landgren O, Gilbert ES, Rizzo JD, Socie G, Banks PM, Sobocinski KA et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood 2009; 113: 4992–5001.
Gottschalk S, Rooney CM, Heslop HE . Post-transplant lymphoproliferative disorders. Annu Rev Med 2005; 56: 29–44.
Svahn A, Berggren J, Parke A, Storsaeter J, Thorstensson R, Linde A . Changes in seroprevalence to four herpesviruses over 30 years in Swedish children aged 9–12 years. J Clin Virol 2006; 37: 118–123.
Takeuchi K, Tanaka-Taya K, Kazuyama Y, Ito YM, Hashimoto S, Fukayama M et al. Prevalence of Epstein–Barr virus in Japan: trends and future prediction. Pathol Int 2006; 56: 112–116.
Williams H, Crawford DH . Epstein–Barr virus: the impact of scientific advances on clinical practice. Blood 2006; 107: 862–869.
Bacigalupo A, Lamparelli T, Milone G, Sormani MP, Ciceri F, Peccatori J et al. Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant 2009; 45: 385–391.
Chiang KY, Hazlett LJ, Godder KT, Abhyankar SH, Christiansen NP, van Rhee F et al. Epstein–Barr virus-associated B cell lymphoproliferative disorder following mismatched related T cell-depleted bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1117–1123.
Shapiro RS, McClain K, Frizzera G, Gajl-Peczalska KJ, Kersey JH, Blazar BR et al. Epstein–Barr virus associated B cell lymphoproliferative disorders following bone marrow transplantation. Blood 1988; 71: 1234–1243.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 2009; 10: 855–864.
Hou HA, Yao M, Tang JL, Chen YK, Ko BS, Huang SY et al. Poor outcome in post transplant lymphoproliferative disorder with pulmonary involvement after allogeneic hematopoietic SCT: 13 years’ experience in a single institute. Bone Marrow Transplant 2009; 43: 315–321.
Juvonen E, Aalto SM, Tarkkanen J, Volin L, Mattila PS, Knuutila S et al. High incidence of PTLD after non-T-cell-depleted allogeneic haematopoietic stem cell transplantation as a consequence of intensive immunosuppressive treatment. Bone Marrow Transplant 2003; 32: 97–102.
Meijer E, Slaper-Cortenbach IC, Thijsen SF, Dekker AW, Verdonck LF . Increased incidence of EBV-associated lymphoproliferative disorders after allogeneic stem cell transplantation from matched unrelated donors due to a change of T cell depletion technique. Bone Marrow Transplant 2002; 29: 335–339.
Knowles DM, Cesarman E, Chadburn A, Frizzera G, Chen J, Rose EA et al. Correlative morphologic and molecular genetic analysis demonstrates three distinct categories of posttransplantation lymphoproliferative disorders. Blood 1995; 85: 552–565.
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010; 115: 925–935.
Styczynski J, Einsele H, Gil L, Ljungman P . Outcome of treatment of Epstein–Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis 2009; 11: 383–392.
Curtis RE, Travis LB, Rowlings PA, Socie G, Kingma DW, Banks PM et al. Risk of lymphoproliferative disorders after bone marrow transplantation: a multi-institutional study. Blood 1999; 94: 2208–2216.
Clave E, Agbalika F, Bajzik V, Peffault de Latour R, Trillard M, Rabian C et al. Epstein–Barr virus (EBV) reactivation in allogeneic stem-cell transplantation: relationship between viral load, EBV-specific T-cell reconstitution and rituximab therapy. Transplantation 2004; 77: 76–84.
Guppy AE, Rawlings E, Madrigal JA, Amlot PL, Barber LD . A quantitative assay for Epstein–Barr virus-specific immunity shows interferon-gamma producing CD8+ T cells increase during immunosuppression reduction to treat posttransplant lymphoproliferative disease. Transplantation 2007; 84: 1534–1539.
Meij P, van Esser JW, Niesters HG, van Baarle D, Miedema F, Blake N et al. Impaired recovery of Epstein–Barr virus (EBV)—specific CD8+ T lymphocytes after partially T-depleted allogeneic stem cell transplantation may identify patients at very high risk for progressive EBV reactivation and lymphoproliferative disease. Blood 2003; 101: 4290–4297.
Russell JA, Turner AR, Larratt L, Chaudhry A, Morris D, Brown C et al. Adult recipients of matched related donor blood cell transplants given myeloablative regimens including pretransplant antithymocyte globulin have lower mortality related to graft-versus-host disease: a matched pair analysis. Biol Blood Marrow Transplant 2007; 13: 299–306.
Russell JA, Savoie ML, Balogh A, Turner AR, Larratt L, Chaudhry MA et al. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400 CGY total-body irradiation, and thymoglobulin. Biol Blood Marrow Transplant 2007; 13: 814–821.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Swerdlow SH, Webber SA, Chadburn A, Ferry JA . Post-transplant lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Harris NL, Stein H, Vardiman JW, Pileri SA, Stein H et al. (eds). WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon, 2008, pp 343–349.
Gasser O, Bihl F, Sanghavi S, Rinaldo C, Rowe D, Hess C et al. Treatment-dependent loss of polyfunctional CD8+ T-cell responses in HIV-infected kidney transplant recipients is associated with herpesvirus reactivation. Am J Transplant 2009; 9: 794–803.
Harari A, Vallelian F, Meylan PR, Pantaleo G . Functional heterogeneity of memory CD4T cell responses in different conditions of antigen exposure and persistence. J Immunol 2005; 174: 1037–1045.
Nash RA, Dansey R, Storek J, Georges GE, Bowen JD, Holmberg LA et al. Epstein–Barr virus-associated posttransplantation lymphoproliferative disorder after high-dose immunosuppressive therapy and autologous CD34-selected hematopoietic stem cell transplantation for severe autoimmune diseases. Biol Blood Marrow Transplant 2003; 9: 583–591.
Storek J, Dawson MA, Storer B, Stevens-Ayers T, Maloney DG, Marr KA et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood 2001; 97: 3380–3389.
Milpied N, Vasseur B, Parquet N, Garnier JL, Antoine C, Quartier P et al. Humanized anti-CD20 monoclonal antibody (Rituximab) in post transplant B-lymphoproliferative disorder: a retrospective analysis on 32 patients. Ann Oncol 2000; 11 (Suppl 1): 113–116.
Kuehnle I, Huls MH, Liu Z, Semmelmann M, Krance RA, Brenner MK et al. CD20 monoclonal antibody (rituximab) for therapy of Epstein–Barr virus lymphoma after hemopoietic stem-cell transplantation. Blood 2000; 95: 1502–1505.
Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC et al. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant 2006; 6: 569–576.
Faye A, Quartier P, Reguerre Y, Lutz P, Carret AS, Dehee A et al. Chimaeric anti-CD20 monoclonal antibody (rituximab) in post-transplant B-lymphoproliferative disorder following stem cell transplantation in children. Br J Haematol 2001; 115: 112–118.
Frassoni F . Anti-T-cell globulin: an essential ingredient for haematopoietic cell transplantation? Lancet Oncol 2009; 10: 839.
Kim HJ, Min WS, Cho BS, Eom KS, Kim YJ, Min CK et al. Successful prevention of acute graft-versus-host disease using low-dose antithymocyte globulin after mismatched, unrelated, hematopoietic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant 2009; 15: 704–717.
Chen XH, Zhang C, Zhang X, Gao L, Kong PY, Peng XG et al. Role of antithymocyte globulin and granulocyte-colony stimulating factor-mobilized bone marrow in allogeneic transplantation for patients with hematologic malignancies. Biol Blood Marrow Transplant 2009; 15: 266–273.
Bredeson CN, Zhang MJ, Agovi MA, Bacigalupo A, Bahlis NJ, Ballen K et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant 2008; 14: 993–1003.
Ayuk F, Diyachenko G, Zabelina T, Panse J, Wolschke C, Eiermann T et al. Anti-thymocyte globulin overcomes the negative impact of HLA mismatching in transplantation from unrelated donors. Exp Hematol 2008; 36: 1047–1054.
Ayuk F, Diyachenko G, Zabelina T, Wolschke C, Fehse B, Bacher U et al. Comparison of two doses of antithymocyte globulin in patients undergoing matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2008; 14: 913–919.
Deeg HJ, Storer BE, Boeckh M, Martin PJ, McCune JS, Myerson D et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant 2006; 12: 573–584.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001; 98: 2942–2947.
Podgorny PJ, Ugarte-Torres A, Liu Y, Williamson TS, Russell JA, Storek J . High rabbit-anti-human thymocyte globulin levels are associated with low likelihood of graft-vs-host disease and high likelihood of posttransplant lymphoproliferative disorder. Biol Blood Marrow Transplant 2010; 16: 915–926.
Meijer E, Cornelissen JJ . Epstein–Barr virus-associated lymphoproliferative disease after allogeneic haematopoietic stem cell transplantation: molecular monitoring and early treatment of high-risk patients. Curr Opin Hematol 2008; 15: 576–585.
Omar H, Hagglund H, Gustafsson-Jernberg A, LeBlanc K, Mattsson J, Remberger M et al. Targeted monitoring of patients at high risk of post-transplant lymphoproliferative disease by quantitative Epstein–Barr virus polymerase chain reaction. Transpl Infect Dis 2009; 11: 393–399.
van Esser JW, Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW et al. Prevention of Epstein–Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood 2002; 99: 4364–4369.
Swinnen LJ . Immune-cell treatment of Epstein–Barr-virus-associated lymphoproliferative disorders. Best Pract Res Clin Haematol 2006; 19: 839–847.
Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA et al. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood 2004; 103: 3979–3981.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009; 43: 757–770.
Zaia J, Baden L, Boeckh MJ, Chakrabarti S, Einsele H, Ljungman P et al. Viral disease prevention after hematopoietic cell transplantation. Bone Marrow Transplant 2009; 44: 471–482.
Hislop AD, Taylor GS, Sauce D, Rickinson AB . Cellular responses to viral infection in humans: lessons from Epstein–Barr virus. Annu Rev Immunol 2007; 25: 587–617.
Acknowledgements
We thank the patients for participating in research that could not benefit them but only future patients. This study could not happen without the dedication of Polly Louie, Lynne Fisk, Judy Wu, Diana Quinlan, Maggie Yang, Glennis Doiron, Dr Doug Demetrick as well as the staff of the Alberta Blood and Marrow Transplant Program, including in-patient and outpatient nurses and physicians, including Drs Ahsan Chaudhry, Nancy Zacarias, Ping Yue, Nizar Bahlis, Chris Brown, Lynn Savoie, Mona Shafey, Loree Larratt and Robert Turner. We also appreciate help from Tyler S Williamson (statistician) and Dr Raymong Tellier and Xiao-Li Pang of Alberta Provincial Laboratory. This work was funded by Alberta Heritage Foundation for Medical Research, Canada Research Chair Program, Alberta Cancer Research Institute and the University of Calgary O’Brien Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Hoegh-Petersen, M., Goodyear, D., Geddes, M. et al. High incidence of post transplant lymphoproliferative disorder after antithymocyte globulin-based conditioning and ineffective prediction by day 28 EBV-specific T lymphocyte counts. Bone Marrow Transplant 46, 1104–1112 (2011). https://doi.org/10.1038/bmt.2010.272
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2010.272
Keywords
This article is cited by
-
Outcomes for patients with EBV-positive PTLD post-allogeneic HCT after failure of rituximab-containing therapy
Bone Marrow Transplantation (2024)
-
Lack of both donor and recipient anti-EBV T cells in EBV seronegative recipients of grafts from seropositive donors
Bone Marrow Transplantation (2023)
-
Epstein-Barr virus-related post-transplant lymphoproliferative disease (EBV-PTLD) in the setting of allogeneic stem cell transplantation: a comprehensive review from pathogenesis to forthcoming treatment modalities
Bone Marrow Transplantation (2020)
-
Hematopoietic stem cell transplantation for adults with EBV-positive T- or NK-cell lymphoproliferative disorders: efficacy and predictive markers
Bone Marrow Transplantation (2016)
-
Greatly reduced risk of EBV reactivation in rituximab-experienced recipients of alemtuzumab-conditioned allogeneic HSCT
Bone Marrow Transplantation (2016)