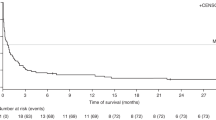
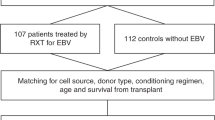
Abstract
To minimize mortality due to posttransplant lymphoproliferative disorder (PTLD), the following strategies have been used: (1) Therapy without EBV Monitoring, i.e., administration of rituximab after PTLD diagnosis, usually by biopsy, in the absence of routine Epstein–Barr virus (EBV) DNAemia monitoring, (2) Prompt Therapy, i.e., monitoring EBV DNAemia, searching for PTLD by imaging when the DNAemia has exceeded a pre-specified threshold, and administration of rituximab if the imaging is consistent with PTLD, (3) Preemptive Therapy, i.e., monitoring EBV DNAemia and administration of rituximab when the DNAemia has exceeded a pre-specified threshold, and (4) Prophylaxis, i.e., administration of rituximab to all transplant recipients. The superiority of one of these strategies over the other strategies has not been established. Here we review the pros and cons of each strategy. Preemptive therapy or prophylaxis may currently be preferred for patients who are at a high risk of dying due to PTLD. However, Therapy without EBV Monitoring may be used for both high- and low-risk patients in the future, if effective and relatively non-toxic therapies for rituximab-refractory PTLD (e.g., EBV-specific T cells) have become easily available.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lee M, Abousaud A, Harkins RA, Marin E, Balasubramani D, Churnetski MC, et al. Important considerations in the diagnosis and management of post-transplant lymphoproliferative disorder. Curr Oncol Rep. 2023;25:883–95. https://doi.org/10.1007/s11912-023-01418-0.
Lindsay J, Othman J, Heldman MR, Slavin MA. Epstein-Barr virus posttransplant lymphoproliferative disorder: update on management and outcomes. Curr Opin Infect Dis. 2021;34:635–45. https://doi.org/10.1097/QCO.0000000000000787.
Styczynski J, Einsele H, Gil L, Ljungman P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis. 2009;11:383–92. https://doi.org/10.1111/j.1399-3062.2009.00411.x.
Coppoletta S, Tedone E, Galano B, Soracco M, Raiola AM, Lamparelli T, et al. Rituximab treatment for Epstein-Barr virus DNAemia after alternative-donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:901–7. https://doi.org/10.1016/j.bbmt.2010.10.003.
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35. https://doi.org/10.1182/blood-2009-08-239186.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN, et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant. 2009;43:757–70. https://doi.org/10.1038/bmt.2008.386.
Yan N, Wang N, Zhang P, Wang G, Mao X, Peng D, et al. Case report: successful chimeric antigen receptor T cell therapy in haploidentical-allogeneic stem cell transplant patients with post-transplant lymphoproliferative disorder. Front Oncol. 2021;11:709370. https://doi.org/10.3389/fonc.2021.709370.
Luttwak E, Hagin D, Perry C, Wolach O, Itchaki G, Amit O, et al. Anti-CD19 CAR-T therapy for EBV-negative posttransplantation lymphoproliferative disease-a single center case series. Bone Marrow Transplant. 2021;56:1031–7. https://doi.org/10.1038/s41409-020-01145-1.
Chen S, An L, Han J, Zheng X, Zhang X, Li G, et al. Successful Blinatumomab treatment in an allogeneic hematopoietic stem cell transplant recipient with EBV-related post-transplant lymphoproliferative disorder: a case report and literature review. Transpl Immunol. 2023;80:101895. https://doi.org/10.1016/j.trim.2023.101895.
Janardan S, Horwitz E, Watkins B, Williams K, Chandrakasan S, Qayed M, et al. Blinatumomab induces complete response in refractory PTLD after hematopoietic cell transplantation. Blood Adv. 2022;6:3058–61. https://doi.org/10.1182/bloodadvances.2021006535.
Cesaro S, Pegoraro A, Tridello G, Calore E, Pillon M, Varotto S, et al. A prospective study on modulation of immunosuppression for Epstein-Barr virus reactivation in pediatric patients who underwent unrelated hematopoietic stem-cell transplantation. Transplantation. 2010;89:1533–40. https://doi.org/10.1097/TP.0b013e3181dd6c0a.
Cesaro S, Murrone A, Mengoli C, Pillon M, Biasolo MA, Calore E, et al. The real-time polymerase chain reaction-guided modulation of immunosuppression enables the pre-emptive management of Epstein-Barr virus reactivation after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2005;128:224–33. https://doi.org/10.1111/j.1365-2141.2004.05287.x.
Prockop S, Mahadeo M, Beitinfaneh A, Chquet S, Stiff P, Reshef R, et al. Multicenter, open-label, phase 3 study of tabelecleucel for solid organ or allogeneic hematopoietic cell transplant recipients with Epstein–Barr virus-driven post transplant lymphoproliferative disease after failure of rituximab or rituximab and chemotherapy (ALLELE). In: American Society of Hematology Annual Meeting. Atlanta, GA, USA, 2021.
Al Hamed R, Bazarbachi AH, Mohty M. Epstein-Barr virus-related post-transplant lymphoproliferative disease (EBV-PTLD) in the setting of allogeneic stem cell transplantation: a comprehensive review from pathogenesis to forthcoming treatment modalities. Bone Marrow Transplant. 2020;55:25–39. https://doi.org/10.1038/s41409-019-0548-7.
Dominietto A, Tedone E, Soracco M, Bruno B, Raiola AM, Van Lint MT, et al. In vivo B-cell depletion with rituximab for alternative donor hemopoietic SCT. Bone Marrow Transplant. 2012;47:101–6. https://doi.org/10.1038/bmt.2011.28.
Blaes AH, Cao Q, Wagner JE, Young JA, Weisdorf DJ, Brunstein CG. Monitoring and preemptive rituximab therapy for Epstein-Barr virus reactivation after antithymocyte globulin containing nonmyeloablative conditioning for umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16:287–91. https://doi.org/10.1016/j.bbmt.2009.10.008.
Kalra A, Roessner C, Jupp J, Williamson T, Tellier R, Chaudhry A, et al. Risk factors for post-transplant lymphoproliferative disorder after Thymoglobulin-conditioned hematopoietic cell transplantation. Clin Transplant. 2018;32. https://doi.org/10.1111/ctr.13150.
van Esser JW, Niesters HG, van der Holt B, Meijer E, Osterhaus AD, Gratama JW, et al. Prevention of Epstein-Barr virus-lymphoproliferative disease by molecular monitoring and preemptive rituximab in high-risk patients after allogeneic stem cell transplantation. Blood. 2002;99:4364–9.
Kinzel M, Dowhan M, Kalra A, Williamson TS, Dabas R, Jamani K, et al. Risk factors for the incidence of and the mortality due to post-transplant lymphoproliferative disorder after hematopoietic cell transplantation. Transpl Cell Ther. 2022;28:53.e1–10. https://doi.org/10.1016/j.jtct.2021.09.021.
Van Besien K, Bachier-Rodriguez L, Satlin M, Brown MA, Gergis U, Guarneri D, et al. Prophylactic rituximab prevents EBV PTLD in haplo-cord transplant recipients at high risk. Leuk Lymphoma. 2019;60:1693–6. https://doi.org/10.1080/10428194.2018.1543877.
Patel C, Pasciolla M, Abramova R, Salerno D, Gomez-Arteaga A, Shore TB, et al. Pre-hematopoietic stem cell transplantation rituximab for Epstein-Barr virus and post-lymphoproliferative disorder prophylaxis in alemtuzumab recipients. Transpl Cell Ther. 2023;29:132.e131–5. https://doi.org/10.1016/j.jtct.2022.10.023.
Kalra A, Roessner C, Jupp J, Williamson T, Tellier R, Chaudhry A, et al. Epstein-Barr virus DNAemia monitoring for the management of post-transplant lymphoproliferative disorder. Cytotherapy. 2018;20:706–14. https://doi.org/10.1016/j.jcyt.2018.02.367.
Juvonen E, Aalto S, Tarkkanen J, Volin L, Hedman K, Ruutu T. Retrospective evaluation of serum Epstein Barr virus DNA levels in 406 allogeneic stem cell transplant patients. Haematologica. 2007;92:819–25.
Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, et al. Response to rituximab-based therapy and risk factor analysis in Epstein Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57:794–802. https://doi.org/10.1093/cid/cit391.
Lindsay J, Othman J, Yong MK, Ritchie D, Chee L, Tay K, et al. Dynamics of Epstein-Barr virus on post-transplant lymphoproliferative disorders after antithymocyte globulin-conditioned allogeneic hematopoietic cell transplant. Transpl Infect Dis. 2021;23:e13719. https://doi.org/10.1111/tid.13719.
Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, et al. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–11. https://doi.org/10.3324/haematol.2016.144428.
Garcia-Cadenas I, Castillo N, Martino R, Barba P, Esquirol A, Novelli S, et al. Impact of Epstein Barr virus-related complications after high-risk allo-SCT in the era of pre-emptive rituximab. Bone Marrow Transplant. 2015;50:579–84. https://doi.org/10.1038/bmt.2014.298.
Pinana JL, Sanz J, Esquirol A, Martino R, Picardi A, Barba P, et al. Umbilical cord blood transplantation in adults with advanced Hodgkin’s disease: high incidence of post-transplant lymphoproliferative disease. Eur J Haematol. 2016;96:128–35. https://doi.org/10.1111/ejh.12557.
McIver Z, Stephens N, Grim A, Barrett AJ. Rituximab administration within 6 months of T cell-depleted allogeneic SCT is associated with prolonged life-threatening cytopenias. Biol Blood Marrow Transplant. 2010;16:1549–56. https://doi.org/10.1016/j.bbmt.2010.05.004.
Kinzel M, Kalra A, Khanolkar RA, Williamson TS, Li N, Khan F, et al. Rituximab toxicity after preemptive or therapeutic administration for post-transplant lymphoproliferative disorder. Transpl Cell Ther. 2023;29:43.e41–8. https://doi.org/10.1016/j.jtct.2022.10.013.
Petropoulou AD, Porcher R, Peffault de Latour R, Xhaard A, Weisdorf D, Ribaud P, et al. Increased infection rate after preemptive rituximab treatment for Epstein-Barr virus reactivation after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2012;94:879–83. https://doi.org/10.1097/TP.0b013e3182664042.
Semenova T, Lupo J, Alain S, Perrin-Confort G, Grossi L, Dimier J, et al. Multicenter evaluation of whole-blood Epstein-Barr viral load standardization using the WHO international standard. J Clin Microbiol. 2016;54:1746–50. https://doi.org/10.1128/JCM.03336-15.
Preiksaitis JK, Pang XL, Fox JD, Fenton JM, Caliendo AM, Miller GG, et al. Interlaboratory comparison of Epstein-Barr virus viral load assays. Am J Transplant. 2009;9:269–79. https://doi.org/10.1111/j.1600-6143.2008.02514.x.
Raberahona M, Wackenheim C, Germi R, Carre M, Bulabois CE, Thiebaut A, et al. Dynamics of Epstein-Barr viral load after hematopoietic stem cell transplantation and effect of preemptive rituximab therapy. Transpl Infect Dis. 2016;18:889–95. https://doi.org/10.1111/tid.12618.
Lenert LA, Markowitz DR, Blaschke TF. Primum non nocere? Valuing of the risk of drug toxicity in therapeutic decision making. Clin Pharm Ther. 1993;53:285–91. https://doi.org/10.1038/clpt.1993.23.
Bhella S, Majhail NS, Betcher J, Costa LJ, Daly A, Dandoy CE, et al. Choosing Wisely BMT: American Society for Blood and Marrow Transplantation and Canadian Blood and Marrow Transplant Group’s List of 5 Tests and Treatments to Question in Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2018;24:909–13. https://doi.org/10.1016/j.bbmt.2018.01.017.
Stocker N, Labopin M, Boussen I, Paccoud O, Bonnin A, Malard F, et al. Pre-emptive rituximab treatment for Epstein-Barr virus reactivation after allogeneic hematopoietic stem cell transplantation is a worthwhile strategy in high-risk recipients: a comparative study for immune recovery and clinical outcomes. Bone Marrow Transplant. 2020;55:586–94. https://doi.org/10.1038/s41409-019-0699-6.
Socie G, Barba P, Barlev A, Sanz J, Garcia-Cadenas I, Chevallier P, et al. Outcomes for patients with EBV-positive PTLD post-allogeneic HCT after failure of rituximab-containing therapy. Bone Marrow Transplant. 2023. https://doi.org/10.1038/s41409-023-02127-9.
Castagna L, Sarina B, Bramanti S, Perseghin P, Mariotti J, Morabito L. Donor lymphocyte infusion after allogeneic stem cell transplantation. Transfus Apher Sci. 2016;54:345–55. https://doi.org/10.1016/j.transci.2016.05.011.
Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010;115:2960–70. https://doi.org/10.1182/blood-2009-08-236356.
Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013;31:39–48. https://doi.org/10.1200/JCO.2011.39.8495.
Kallay K, Kassa C, Reti M, Karaszi E, Sinko J, Goda V, et al. Early experience with CliniMACS Prodigy CCS (IFN-gamma) system in selection of virus-specific T cells from third-party donors for pediatric patients with severe viral infections after hematopoietic stem cell transplantation. J Immunother. 2018;41:158–63. https://doi.org/10.1097/CJI.0000000000000197.
Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–56. https://doi.org/10.1182/blood-2011-08-371971.
Prockop S, Doubrovina E, Suser S, Heller G, Barker J, Dahi P, et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J Clin Invest. 2020;130:733–47. https://doi.org/10.1172/JCI121127.
Sanz J, Storek J, Socié G, Thirumalai D, Guzman-Becerra N, Xun P, et al. Clinical outcomes of patients with Epstein–Barr virus-driven post-transplant lymphoproliferative disease following hematopoietic stem cell transplantation who fail rituximab: a multinational, retrospective chart review study. In: American Society of Hematology. Atlanta, GA, 2021. Abstract No. 1454.
Faye A, Quartier P, Reguerre Y, Lutz P, Carret AS, Dehee A, et al. Chimaeric anti-CD20 monoclonal antibody (rituximab) in post-transplant B-lymphoproliferative disorder following stem cell transplantation in children. Br J Haematol. 2001;115:112–8.
Zhu CY, Zhao SS, Wang XK, Wang L, Wang FY, Fang S, et al. Outcome of rituximab-based treatment for post-transplant lymphoproliferative disorder after allogeneic hematopoietic stem cell transplantation: a single-center experience. Ann Transplant. 2019;24:175–84. https://doi.org/10.12659/AOT.914101.
Luo XY, Mo XD, Xu LP, Zhang XH, Wang Y, Liu KY, et al. A retrospective analysis on anti-CD20 antibody-treated Epstein-Barr virus-related posttransplantation lymphoproliferative disorder following ATG-based haploidentical T-replete hematopoietic stem cell transplantation. Ann Hematol. 2020;99:2649–57. https://doi.org/10.1007/s00277-020-04003-8.
Wagner HJ, Cheng YC, Huls MH, Gee AP, Kuehnle I, Krance RA, et al. Prompt versus preemptive intervention for EBV lymphoproliferative disease. Blood. 2004;103:3979–81. https://doi.org/10.1182/blood-2003-12-4287.
Kinch A, Oberg G, Arvidson J, Falk KI, Linde A, Pauksens K. Post-transplant lymphoproliferative disease and other Epstein-Barr virus diseases in allogeneic haematopoietic stem cell transplantation after introduction of monitoring of viral load by polymerase chain reaction. Scand J Infect Dis. 2007;39:235–44. https://doi.org/10.1080/00365540600978906.
Sanz J, Arango M, Senent L, Jarque I, Montesinos P, Sempere A, et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant. 2014;49:397–402. https://doi.org/10.1038/bmt.2013.190.
Garcia-Cadenas I, Yanez L, Jarque I, Martino R, Perez-Simon JA, Valcarcel D, et al. Frequency, characteristics, and outcome of PTLD after allo-SCT: A multicenter study from the Spanish group of blood and marrow transplantation (GETH). Eur J Haematol. 2019;102:465–71. https://doi.org/10.1111/ejh.13226.
Ahmad I, Cau NV, Kwan J, Maaroufi Y, Meuleman N, Aoun M, et al. Preemptive management of Epstein-Barr virus reactivation after hematopoietic stem-cell transplantation. Transplantation. 2009;87:1240–5. https://doi.org/10.1097/TP.0b013e31819f1c49.
Enok Bonong PR, Zahreddine M, Buteau C, Duval M, Laporte L, Lacroix J, et al. Factors associated with post-transplant active Epstein-Barr virus infection and lymphoproliferative disease in hematopoietic stem cell transplant recipients: a systematic review and meta-analysis. Vaccines (Basel). 2021;9:288. https://doi.org/10.3390/vaccines9030288.
Marjanska A, Styczynski J. Who is the patient at hisk fo EBV reactivation and diseases: expert opinion focused on post-transplant lymphoproliferative disorders following hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2023;23:539–52.
Author information
Authors and Affiliations
Contributions
JS wrote the first draft and JL critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Storek, J., Lindsay, J. Rituximab for posttransplant lymphoproliferative disorder – therapeutic, preemptive, or prophylactic?. Bone Marrow Transplant 59, 6–11 (2024). https://doi.org/10.1038/s41409-023-02155-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-02155-5