Abstract
Background:
We aimed to assess the efficacy of second-line fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer (BTC) after failure of gemcitabine plus cisplatin (GEMCIS).
Methods:
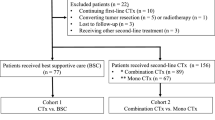
We retrospectively examined patients with histologically documented advanced BTC who received first-line GEMCIS between December 2010 and June 2015. Among 748 patients treated with first-line GEMCIS, 321 (43%) subsequently received fluoropyrimidine-based second-line systemic chemotherapy.
Results:
Fluoropyrimidine monotherapy and fluoropyrimidine–platinum combination were used in 255 and 66 patients, respectively. In patients with measurable disease, the overall response rate (ORR) was 3% and disease control rate was 47%. After a median follow-up of 27.6 months (range, 0.9–70.4 months), the median progression-free survival (PFS) and overall survival (OS) were 1.9 months (95% confidence interval (CI), 1.6–2.2) and 6.5 months (95% CI, 5.9–7.0), respectively. The ORR was significantly higher in patients who received fluoropyrimidine–platinum combination compared with those who received fluoropyrimidine alone (8 vs 1%, P=0.009), although the PFS (P=0.43) and OS (P=0.88) did not significantly differ between these groups.
Conclusions:
Fluoropyrimidine-based chemotherapy was modestly effective as a second-line chemotherapy for advanced BTC patients after failure of GEMCIS. Fluoropyrimidine–platinum combination therapy was not associated with improved survival outcomes, as compared with fluoropyrimidine monotherapy.
Similar content being viewed by others
Main
Biliary tract cancer (BTC) is a heterogeneous group of diseases that include intrahepatic/extrahepatic cholangiocarcinoma and gallbladder cancer. It is a rare malignancy, and ∼10 000 new cases are diagnosed annually in the United states and Europe (Siegel et al, 2014). In Korea, crude incidence rate of BTC was reported to reach 11.4 patients per 100 000 population in 2016 (Jung et al, 2016). Although surgical resection is the only curative treatment modality for localised disease, most patients experience disease recurrence even after complete resection; moreover, the 5-year overall survival (OS) rates of advanced BTC is ∼10%, and hence prognosis is poor (Edge and Compton, 2010).
As the randomised phase III ABC-02 trial indicated that gemcitabine plus cisplatin (GEMCIS) yields significantly improved overall survival (OS), as compared with gemcitabine alone (11.7 vs 8.1 months), the GEMCIS regimen has been globally accepted as the standard first-line chemotherapy for patients with unresectable or metastatic BTC (Valle et al, 2010). Eventually, most patients experience disease progression, despite GEMCIS treatment, and subsequent chemotherapy may help prolong survival and maintain the quality of life, at least in medically fit patients after GEMCIS failure. Previous studies showed that ∼50% of patients still have good performance status after first-line chemotherapy failure and may serve as candidates for second-line chemotherapy (Kim et al, 2008). Recent advances in the supportive care, particularly biliary drainage procedures, may enable a greater number of patients to receive subsequent active anticancer treatment after disease progression despite the application of first-line chemotherapy.
The role of second-line chemotherapy in advanced BTC remains unclear. To our knowledge, no randomised study has been performed to indicate the survival benefit of second-line chemotherapy over best supportive care. Despite the lack of level 1 evidence, second-line chemotherapy has been widely used in clinical practice for patients with advanced BTC (Ducreux et al, 2005; Pino et al, 2009; Sasaki et al, 2009, 2012; Kobayashi et al, 2012; Lim et al, 2012; Yi et al, 2012; Bridgewater et al, 2013; Cereda et al, 2013; Suzuki et al, 2013; Walter et al, 2013; Lamarca et al, 2014; Fiteni et al, 2014; Fornaro et al, 2014, 2015; Brieau et al, 2015). Although these studies have examined the efficacy and safety of second-line chemotherapy in advanced BTC, most were retrospective studies based on a small sample size and included diverse types of first-line chemotherapy. Hence, more data are needed to evaluate the efficacy of second-line chemotherapy in a large patient population that received the same first-line treatment. Such analysis will also be important for designing future clinical trials that investigate the outcomes of second-line chemotherapy after GEMCIS failure, considering the heterogeneous characteristics of advanced BTC.
In the present study, we retrospectively assessed the efficacy of second-line chemotherapy in patients with advanced BTC after the failure of first-line GEMCIS treatment. Moreover, switching to a fluoropyrimidine-based regimen is generally considered clinically appropriate in patients with disease progression on first-line gemcitabine–platinum combination therapy (Lamarca et al, 2014), despite this not being validated in the prospective trial. Hence, we evaluated the clinical outcomes of fluoropyrimidine-based regimens and prognostic factors in the setting of second-line chemotherapy.
Materials and methods
Patients
Patients with histologically confirmed advanced BTC who received first-line GEMCIS chemotherapy at Asan Medical Center, Seoul, Korea, between April 2010 and June 2015, were identified, and their medical records were retrospectively reviewed; patients with ampullary tumour were not included. Among 748 patients treated with first-line GEMCIS, 331 (44%) subsequently received second-line systemic chemotherapy, including fluoropyrimidine-based chemotherapy in 321 patients (97%). The following information was extracted from the medical records of each eligible patient: demographics, tumour characteristics, performance status at presentation, best response to GEMCIS, time to tumour progression (TTP) from GEMCIS initiation, CA 19-9 level at presentation, date of disease progression and survival status at the last follow-up.
The tumour response was assessed at 6- or 8-week intervals using computed tomography or magnetic resonance imaging, and was graded according to the Response Evaluation Criteria in Solid Tumours version 1.1 (Eisenhauer et al, 2009). The Institutional Review Board of Asan Medical Center approved this study and waived the requirement for informed consent.
Statistical analysis
Progression-free survival (PFS) was defined as the duration from the initiation of the second-line chemotherapy to disease progression or death, whichever occurred first. OS was defined as the duration from the initiation of second-line chemotherapy and any cause of death. Categorical variables were compared using χ2 or Fisher’s exact tests, as appropriate. Overall survival and PFS curves were estimated using the Kaplan–Meier method and compared using log-rank tests. Univariate and multivariate analyses were performed to identify the prognostic factors for PFS and OS based on the Cox proportional hazard model with inclusion of variables that may affect the prognosis (sex, age, primary tumour site, disease extent, response to first-line GEMCIS, performance status, CA 19-9 level and second-line regimen). Multivariate analysis was performed using Cox proportional hazard model developed with backward likelihood ratio method. Key patients’ characteristics, such as sex and age, and the variables that showed a potential prognostic significance (P<0.10) in the univariate analyses were included in the multivariate analyses. Two-sided P-values <0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS, Chicago, IL, USA) version 21.0.
Results
Patient characteristics
The baseline characteristics of the patients are summarised in Table 1. The median age was 60 years (range, 27–82 years), and 57% of patients were male. The intrahepatic region was the most common primary tumour site (44%), followed by the extraheptic region (32%) and gallbladder (24%). Most of the patients had metastatic or recurrent disease (89%), or had an Eastern Cooperative Oncology Group performance status of 0 or 1 (91%) at the time of first-line GEMCIS. The liver (41%) and intra-abdominal lymph nodes (41%) were the most common metastatic sites. The CA 19-9 levels were elevated in 51% of patients at the time of first-line GEMCIS.
Outcomes of first-line GEMCIS
All the patients were treated using the GEMCIS dosing schedule described in the pivotal ABC-02 trial. Partial response and stable disease were achieved in 9% (30 out of 321) and 59% (188/321) of patients, respectively. The median TTP to first-line GEMCIS was 4.2 months (95% confidence interval (CI), 3.5–5.0 months).
Second-line fluoropyrimidine-based chemotherapy
Fluoropyrimidine monotherapy was used in in 79% (255 out of 321) of patients as second-line chemotherapy, including infusional 5-fluorouracil/leucovorin in 133, S-1 in 111, UFT/leucovorin in 7 and capecitabine in 4. The other patients (21%, n=66) received a combination of fluoropyrimidine and platinum, including capecitabine plus cisplatin in 60, 5-fluorouracil plus cisplatin in 2, 5-fluorouracil plus oxaliplatin in 2 and capecitabine plus oxaliplatin in 2.
None of the patients with available response assessments exhibited a complete response. Overall, complete/partial response and disease control (complete/partial response plus stable disease) were achieved in 8 (2%) and 142 (44%) patients, respectively (Table 2). The response rate was significantly higher in patients who received fluoropyrimidine–platinum combination, as compared with those who received fluoropyrimidine monotherapy (8% vs 1%, P=0.009). Although the response rates were higher in patients with gallbladder cancer (4%) compared with those with intrahepatic (2%) and extrahepatic cholangiocarcinoma (2%), the difference was not significant (P=0.66).
Over a median follow-up duration of 27.6 months (range, 0.9–70.4 months), the median PFS and OS with the second-line fluoropyrimidine-based chemotherapy were found to be 1.9 months (95% CI, 1.6–2.2 months) and 6.5 months (95% CI, 5.9–7.0 months), respectively. There were no significant differences between fluoropyrimidine monotherapy and fluoropyrimidine-platinum in terms of PFS (median, 1.8 vs 2.6 months; P=0.43) and OS (median, 6.5 vs. 6.2 months; P=0.87; Figure 1).
Prognostic factor analysis
Univariate and multivariate analyses were performed to define the prognostic factors in patients who received second-line chemotherapy. Intrahepatic cholangiocarcinoma was the only significant factor associated with poorer PFS (vs gallbladder cancer: 1.6 months (95% CI, 1.6–1.7 months) vs 3.2 (95% CI, 2.7–3.7 months); hazard ratio (HR), 1.65 (1.17–2.32); P=0.004) on multivariate analysis (Table 3). Prolonged TTP from first-line GEMCIS initiation showed potential association with favourable PFS on univariate analysis (>4 months vs ⩽4 months: 2.5 months (95% CI, 1.9–3.2) vs 1.8 months (1.6–1.9 months)), although only a marginal association was observed on multivariate analysis (HR, 0.79 (95% CI, 0.61–1.04); P=0.09).
Multivariate analysis for OS (Table 4) indicated that intrahepatic cholangiocarcinoma (vs gallbladder cancer: 5.3 months (95% CI, 4.5–6.0 months) vs 7.7 months (95% CI, 6.7–8.6 months); HR, 1.52 (1.08–2.13); P=0.02) and elevated CA 19-9 levels at presentation (vs normal values: 6.3 months (95% CI, 5.5–7.1 months) vs 7.6 months (95% CI, 6.3–9.0 months); HR, 1.50 (1.13–1.98); P=0.005) were significantly associated with poor prognosis. Prolonged TTP from first-line GEMCIS initiation was associated with better OS (>4 months vs ⩽4 months: 7.5 months (95% CI, 6.6–8.7 months) vs 5.6 (95% CI, 4.7–6.4 months); HR, 0.57 (0.43–0.74); P<0.001). Although the disease setting at presentation was not associated with PFS, it was significantly associated with OS. Initially metastatic disease showed poorer OS (median, 4.9 months (95% CI, 4.2–5.6 months)), as compared with locally advanced disease (median, 6.5 months (95% CI, 4.3–8.8 months); HR, 0.50 (0.31–0.82); P=0.005) and recurrent disease after surgery (median, 7.8 months (95% CI, 6.1–9.4 months); HR, 0.62 (0.45–0.85); P=0.003).
In the multivariate models that included potential confounding factors for the outcomes of chemotherapy, the fluoropyrimidine–platinum combination did not show a relationship with better clinical outcomes, although there were marginal associations with PFS (HR, 0.75 (95% CI, 0.52–1.10); P=0.14) and OS (HR, 0.70 (95% CI, 0.46–1.06); P=0.09).
Discussion
In the present study, 44% of patients who received first-line GEMCIS subsequently received second-line chemotherapy. As fluoropyrimidine-based chemotherapy was considered a clinically reasonable option in daily practice, it was administered to most patients (97%) who received second-line chemotherapy. We found that fluoropyrimidine-based chemotherapy was modestly effective as a second-line chemotherapy in advanced BTC patients after the failure of first-line GEMCIS. Although higher response rates were noted, fluoropyrimidine–platinum combination therapy was not associated with improved survival outcomes, as compared with fluoropyrimidine monotherapy. Intrahepatic primary tumour location, elevated CA 19-9 levels, metastatic disease at initial presentation and rapid progression during previous GEMCIS treatment were identified as factors of poor prognosis.
In the present study, the median PFS and OS of second-line fluoropyrimidine-based chemotherapy were 1.9 and 6.5 months, respectively. Our results are consistent with those of previous studies, wherein the median PFS and OS were found to be 3–4 and 6–7 months, respectively (Fornaro et al, 2014; Lamarca et al, 2014; Brieau et al, 2015). Although the PFS outcomes in our cohort appeared to be poorer than those in previous studies, it should be noted that our analysis was performed on an unselected patient population, unlike prospective studies, and that BTC may have heterogeneous clinical features according to the primary tumour site.
In the present study, fluoropyrimidine–platinum combination therapy was associated with higher response rates, as compared with fluoropyrimidine monotherapy (8% vs 1%). However, this did not translate into significant improvements in the PFS (median, 2.6 months vs 1.8 months) or OS (median, 6.2 months vs 6.5 months). This finding was also noted in multivariate analyses in which the impact of potential confounding factors was adjusted. Our results are supported by the recent multicentre retrospective analysis of 196 patients who received second-line chemotherapy after the failure of gemcitabine–platinum combination (Brieau et al, 2015). In this study (Brieau et al, 2015), the median OS with fluoropyrimidine monotherapy and combination treatment were 5.6 and 6.3 months (P=0.93), respectively. However, the lack of benefit of using combination regimens as a second-line chemotherapy cannot be concluded at present, as a previous multicentre survey analysis indicated potential benefit in terms of OS with combination chemotherapy, as compared with monotherapy, despite the absence of any benefit in terms of PFS (Fornaro et al, 2015). The lack of benefit in terms of survival outcome with fluoropyrimidine–platinum combination in the present study could be attributed to the fact that most patients (91%, 60 out of 66) were readministered cisplatin as a partner of fluoropyrimidine, considering that all the patients in this study were already exposed to cisplatin and that the prolonged use of cisplatin may be intolerable in fragile patients after disease progression on first-line therapy. Although oxaliplatin is commonly used globally in the management of advanced BTC, it has not been approved for the treatment of BTC patients in Korea. Therefore, oxaliplatin could be given only in few patients of our cohort.
Because of these conflicting results, further prospective studies are needed to define whether combination therapy is better than monotherapy, or to identify which agent is the optimal partner for the fluoropyrimidine backbone in second-line settings in advanced BTC patients. The ongoing randomised phase III ABC-06 trial comparing modified FOLFOX with best supportive care in the second-line setting may help to measure the efficacy of oxaliplatin–fluoropyrimidine combination. In addition, considering that the patients with advanced BTC after failure of first-line chemotherapy showed a dismal prognosis even with second-line treatment, more efforts are needed to develop novel agents based on the better understanding of biologic features of BTC.
Intrahepatic primary tumour site, elevated CA 19-9 level, metastatic disease at initial presentation and shorter TTP at first-line GEMCIS were poor prognostic factors for patients in second-line settings. These findings were consistent with the results of previous retrospective studies that included a relatively large number of patients. These studies suggest that high CA 19-9 level, metastatic disease at initial presentation and poor response to first-line chemotherapy (no objective response or poor TTP) were independent prognostic factors for OS (Fornaro et al, 2014; Brieau et al, 2015). Given that BTC is heterogeneous in terms of its natural course and molecular characteristics (Nakamura et al, 2015), these prognostic factors should be carefully considered when interpreting the results of prospective studies and designing future clinical trials.
To our knowledge, our current retrospective analysis includes the largest number of patients to date for a study on this topic. Compared with previous studies that included patients with various first-line chemotherapy regimens, our study population is homogenous in terms of that all patients received first-line GEMCIS based on the ABC-02 trial. However, the study design was retrospective in nature and conducted at a single centre, which could have introduced bias. Moreover, our analysis was limited to assessing the impact of CA 19-9 level and performance status in the second-line settings, as only these values were measured at the start of first-line chemotherapy. Serum CA 19-9 levels were not subsequently measured in most patients if the levels were not elevated at the time of initiation of first-line chemotherapy, and the performance status at the time of second-line therapy could also not be accurately estimated because of the retrospective nature of our present analysis.
In conclusion, fluoropyrimidine-based chemotherapy is modestly effective as a second-line chemotherapy after the failure of standard GEMCIS chemotherapy. The clinical implication of second-line chemotherapy in advanced BTC will be clarified in the ongoing ABC-06 phase III randomised trial, which aims to compare best supportive care and modified FOLFOX. However, there is still a lack of evidence regarding which regimen is most effective and tolerable after the failure of GEMCIS, as most previous studies were performed retrospectively and did not include a randomised trial design. Hence, further prospective trials, particularly with a randomised design, are needed to refine the second-line chemotherapy in patients with advanced BTC.
Change history
28 February 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Bridgewater J, Palmer D, Cunningham D, Iveson T, Gillmore R, Waters J, Harrison M, Wasan H, Corrie P, Valle J (2013) Outcome of second-line chemotherapy for biliary tract cancer. Eur J Cancer 49: 1511.
Brieau B, Dahan L, De Rycke Y, Boussaha T, Vasseur P, Tougeron D, Lecomte T, Coriat R, Bachet J-B, Claudez P, Zaanan A, Soibinet P, Desrame J, Thirot-Bidault A, Trouilloud I, Mary F, Marthey L, Taieb J, Cacheux W, Lièvre A (2015) Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 121: 3290–3297.
Cereda S, Belli C, Rognone A, Mazza E, Reni M (2013) Second-line therapy in advanced biliary tract cancer: what should be the standard? Crit Rev Oncol Hematol 88: 368–374.
Ducreux M, Van Cutsem E, Van Laethem JL, Gress TM, Jeziorski K, Rougier P, Wagener T, Anak O, Baron B, Nordlinger B EORTC Gastro Intestinal Tract Cancer Group (2005) A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer 41: 398–403.
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17: 1471–1474.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45: 228–247.
Fiteni F, Jary M, Monnien F, Nguyen T, Beohou E, Demarchi M, Dobi E, Fein F, Cleau D, Fratté S, Nerich V, Bonnetain F, Pivot X, Borg C, Kim S (2014) Advanced biliary tract carcinomas: a retrospective multicenter analysis of first and second-line chemotherapy. BMC Gastroenterol 14: 143.
Fornaro L, Cereda S, Aprile G, Di Girolamo S, Santini D, Silvestris N, Lonardi S, Leone F, Milella M, Vivaldi C, Belli C, Bergamo F, Lutrino SE, Filippi R, Russano M, Vaccaro V, Brunetti AE, Rotella V, Falcone A, Barbera MA, Corbelli J, Fasola G, Aglietta M, Zagonel V, Reni M, Vasile E, Brandi G (2014) Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer 110: 2165–2169.
Fornaro L, Vivaldi C, Cereda S, Leone F, Aprile G, Lonardi S, Silvestris N, Santini D, Milella M, Caparello C, Musettini G, Pasquini G, Falcone A, Brandi G, Sperduti I, Vasile E (2015) Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res 34: 156–162.
Jung K-W, Won Y-J, Oh C-M, Kong H-J, Cho H, Lee J-K, Lee DH, Lee KH (2016) Prediction of cancer incidence and mortality in Korea, 2016. Cancer Res Treat 48: 451–457.
Kim M-J, Oh D-Y, Lee S-H, Kim D-W, Im S-A, Kim T-Y, Heo DS, Bang Y-J (2008) Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer 8: 374.
Kobayashi S, Ueno M, Ohkawa S, Andou T, Kameda R, Yamamoto N, Morinaga S (2012) A retrospective study of S-1 monotherapy as second-line treatment for patients with advanced biliary tract cancer. Jpn J Clin Oncol 42: 800–806.
Lamarca A, Hubner RA, Ryder WD, Valle JW (2014) Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 25: 2328–2338.
Lim K-H, Han S-W, Oh D-Y, Im S-A, Kim T-Y, Bang Y-J (2012) Outcome of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) chemotherapy and analysis of prognostic factors in patients with refractory advanced biliary tract cancer. Oncology 83: 57–66.
Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, Hiraoka N, Ojima H, Shimada K, Okusaka T, Kosuge T, Miyagawa S, Shibata T (2015) Genomic spectra of biliary tract cancer. Nat Genet 47: 1003–1010.
Pino MS, Milella M, Gelibter A, Sperduti I, De Marco S, Nuzzo C, Bria E, Carpanese L, Ruggeri EM, Carlini P, Cognetti F (2009) Capecitabine and celecoxib as second-line treatment of advanced pancreatic and biliary tract cancers. Oncology 76: 254–261.
Sasaki T, Isayama H, Nakai Y, Mizuno S, Yamamoto K, Yagioka H, Yashima Y, Kawakubo K, Kogure H, Togawa O, Matsubara S, Ito Y, Sasahira N, Hirano K, Tsujino T, Toda N, Tada M, Omata M, Koike K (2012) Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs 30: 708–713.
Sasaki T, Isayama H, Yashima Y, Yagioka H, Kogure H, Arizumi T, Togawa O, Matsubara S, Ito Y, Nakai Y, Sasahira N, Hirano K, Tsujino T, Tada M, Kawabe T, Omata M (2009) S-1 monotherapy in patients with advanced biliary tract cancer. Oncology 77: 71–74.
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9–29.
Suzuki E, Ikeda M, Okusaka T, Nakamori S, Ohkawa S, Nagakawa T, Boku N, Yanagimoto H, Sato T, Furuse J (2013) A multicenter phase II study of S-1 for gemcitabine-refractory biliary tract cancer. Cancer Chemother Pharmacol 71: 1141–1146.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J ABC-02 Trial Investigators (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362: 1273–1281.
Walter T, Horgan AM, McNamara M, McKeever L, Min T, Hedley D, Serra S, Krzyzanowska MK, Chen E, Mackay H, Feld R, Moore M, Knox JJ (2013) Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 49: 329–335.
Yi JH, Thongprasert S, Lee J, Doval DC, Park SH, Park JO, Park YS, Kang WK, Lim HY (2012) A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer 48: 196–201.
Acknowledgements
This study was supported in part by the Bio and Medical Technology Development Program of the NRF funded by the Korean government, MSIP (NRF-2016M3A9E8941331).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Kim, B., Yoo, C., Kim, Kp. et al. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: retrospective analysis of 321 patients. Br J Cancer 116, 561–567 (2017). https://doi.org/10.1038/bjc.2016.446
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.446
Keywords
This article is cited by
-
A Systematised Literature Review of Real-World Treatment Patterns and Outcomes in Unresectable Advanced or Metastatic Biliary Tract Cancer
Targeted Oncology (2023)
-
Efficacy and safety of modified FOLFIRINOX as salvage therapy for patients with refractory advanced biliary tract cancer: a retrospective study
Investigational New Drugs (2021)
-
Criteria for liver resection for metastasis from bile duct cancer
Surgery Today (2021)
-
Efficacy and safety of FOLFIRINOX as salvage treatment in advanced biliary tract cancer: an open-label, single arm, phase 2 trial
British Journal of Cancer (2020)
-
Cholangiocarcinoma: State of the Art
Journal of Gastrointestinal Cancer (2020)