Abstract
Background:
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are commonly used antihypertensives. Recently, these drugs have been associated with a protective effect against pancreatic cancer, but data on this putative association remain limited. Thus, the objective of this study was to determine whether the use of ACEIs and/or ARBs is associated with a decreased risk of pancreatic cancer.
Methods:
We conducted a population-based cohort study, using a nested case–control analysis within the UK Clinical Practice Research Datalink population. The cohort consisted of all patients newly treated with antihypertensive drugs between 1 January 1995 and 31 December 2009, with follow-up until 31 December 2010. Cases were patients with newly diagnosed pancreatic cancer, which were matched with up to 10 controls on age, sex, calendar year of cohort entry, and duration of follow-up. Conditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of pancreatic cancer incidence associated with ever use of ACEIs and ARBs. A secondary analysis was conducted to assess whether the incidence of pancreatic cancer varied with cumulative duration of use of these drugs.
Results:
A cohort of 547 566 was assembled. During 3 040 332 person-years of follow-up, a total of 866 patients were newly diagnosed with pancreatic cancer (rate: 3/10 000 per year) and matched to 8636 controls. Overall, when compared with other antihypertensive drugs, the use of ACEIs was not associated with a decreased risk of pancreatic cancer overall (OR: 1.01, 95% CI: 0.86–1.17) or according to cumulative duration of use. The use of ARBs was not associated with a decreased risk of pancreatic cancer overall (OR: 0.93, 95% CI: 0.75–1.15), whereas a cumulative duration of use of 1–3 years was associated with a 38% decrease (OR: 0.62, 95% CI: 0.41–0.94), which returned to the null after >3 years of use (OR: 1.04, 95% CI: 0.74–1.46).
Conclusions:
The use of ARBs and ACEIs was not associated with an overall decreased risk of pancreatic cancer when compared with patients using other antihypertensive drugs. Additional research is needed to determine whether ARBs may confer a short-term protective effect.
Similar content being viewed by others
Main
The role of the renin–angiotensin system (RAS) in the homoeostasis of blood pressure, electrolytes, and volume status is well established (Peach, 1977). There is also evidence that the RAS exists in other tissues, such as the pancreas where it is thought to mediate growth and may have a role in carcinogenesis (Fujimoto et al, 2001; Lam and Leung, 2002; Anandanadesan et al, 2008; Perez-Diaz et al, 2011; Chauhan et al, 2013). Although the exact role of RAS in carcinogenesis remains to be elucidated, it is thought to be implicated in several pathways that may lead to cancer via angiogenesis, inhibition of apoptosis, and influence on stromal cells (Fujimoto et al, 2001; Arafat et al, 2007; Anandanadesan et al, 2008; Chauhan et al, 2013; Masamune et al, 2013).
Given the involvement of RAS in the development of pancreatic cancer, it is biologically plausible that drugs that interfere with this system may have chemopreventive effects. Indeed, angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are two drug classes used in the treatment of hypertension via the blockade of RAS whose use may decrease the risk of pancreatic cancer. In line with this hypothesis, in vitro and animal studies have shown that the use of ACEIs or ARBs inhibited tumour growth by inhibiting angiogenesis or inducing apoptosis (Fujimoto et al, 2001; Arafat et al, 2007; Noguchi et al, 2009; Doi et al, 2010; Fendrich et al, 2010; Arnold et al, 2012; Chauhan et al, 2013; Masamune et al, 2013). Furthermore, it has been demonstrated that hypertension itself may be protective against pancreatic cancer (Eijgenraam et al, 2013). It may be postulated that this effect may be driven by the use of antihypertensive drugs such as ACEIs and ARBs. However, other studies have failed to show an association between hypertension and pancreatic cancer (Stolzenberg-Solomon et al, 2002; Rosato et al, 2011). This is possibly due to the influence of certain antihypertensive drugs.
To date, no observational study has been conducted to assess whether there is an association between the use of ACEIs or ARBs on the incidence of pancreatic cancer. Thus, the objective of this large population-based study was to determine whether the use of ACEIs or ARBs is associated with a decreased risk of pancreatic cancer in patients newly treated with antihypertensive drugs.
Materials and methods
Data source
We conducted a retrospective population-based cohort study, using a nested case–control analysis using the United Kingdom Clinical Practice Research Datalink (CPRD) database. The CPRD includes over 13 million patients followed in general practice in over 680 offices in the United Kingdom. The CPRD records demographic data, diagnoses, procedures, and prescriptions written by general practitioners The information recorded in the CPRD has been shown to have excellent validity (Garcia Rodriguez and Perez Gutthann, 1998; Lawrenson et al, 1999; Jick et al, 2003; Herrett et al, 2010).
The study protocol was approved by the Independent Scientific Advisory Committee of the CPRD (protocol number 15_038R) and by the Research Ethics Board of the Jewish General Hospital, Montreal, Canada.
Study cohort
The cohort consisted of all patients newly treated with antihypertensive drugs (diuretics, beta-blockers, ACEIs, ARBs, calcium channel blockers (CCBs), alpha-adrenergic receptor blocking drugs (alpha-blockers), and other antihypertensives) between 1 January 1995 and 31 December 2009, with follow-up until 31 December 2010. Cohort entry was the date of the first-ever prescription for an antihypertensive drug. To be included in the study, patients were required to have at least 1 year of medical history in the CPRD prior to cohort entry and to have at least 1 year of follow-up after cohort entry, which was necessary for latency considerations. Patients with a history of cystic fibrosis at any time prior to cohort entry were excluded as this is considered to be a risk factor for chronic pancreatitis and thus pancreatic cancer as well (Becker et al, 2014).
Thus, all patients meeting the study inclusion criteria were followed until a first-ever diagnosis of pancreatic cancer, death from any cause, end of registration with the general practice, or end of the study period (31 December 2010), whichever occurred first.
Case–control selection
All patients newly diagnosed with pancreatic cancer during follow-up were identified on the basis of Read codes (available in Supplementary Material, Appendix II). The event date of each case’s pancreatic cancer diagnosis was defined as their index date.
Using risk set sampling, up to 10 controls were randomly selected and matched to each case on age, sex, calendar year of cohort entry, and duration of follow-up. The matched controls inherited the index date of their respective cases. Thus, by definition, all controls were alive, not previously diagnosed with pancreatic cancer, and registered with their general practice when matched to a given case, and thus had equal duration of follow-up at the index date.
Exposure assessment
For all cases and matched controls, we obtained information on the antihypertensive prescriptions received between cohort entry and index date. These consisted of diuretics, beta-blockers, ACEIs, ARBs, CCBs, alpha-blockers, and other antihypertensive drugs.
The use of ACEIs or ARBs was entered as two non-mutually exclusive exposure categories in the models. Ever use was defined as receiving at least one prescription for an ACEI or ARB at any time but excluding the year before index date. The exclusion of exposures initiated in the year immediately prior to index date was to account for a biologically meaningful latency time window, as it was unlikely that a drug would have an effect on the incidence of pancreatic cancer after short exposure durations. This was considered the primary exposure definition.
We also considered four secondary exposure definitions. The first three definitions assessed whether there was a duration-response relationship in terms of cumulative duration of use, number of prescriptions received, and time since initiation. Thus, for patients deemed to be ever users ACEIs or ARBs, we calculated their cumulative duration of use as the sum of all prescription durations received between cohort entry and the index date. Similarly, number of prescriptions received was calculated by summing all prescriptions received between cohort entry and index date. Time since initiation was calculated as time between the first prescription and index date. These three secondary exposure definitions were categorised into tertiles based on the distribution of use in the controls. Finally, the fourth definition reclassified ever use of ACEIs or ARBs into three mutually exclusive categories: ACEIs alone, ARBs alone, and ever use of both ACEIs and ARBs.
Statistical analysis
The overall incidence rate of pancreatic cancer was estimated by dividing the total number of cases by the total person-years of follow-up, with 95% confidence intervals (CIs) based on the Poisson distribution. Conditional logistic regression was used to estimate odds ratios (ORs) with 95% CIs of pancreatic cancer associated with ever use of ACEIs or ARBs, when compared with never use. We also assessed whether there was a duration- and dose–response relationship in terms of cumulative duration, number of prescriptions, and time since initiation. Finally, we assessed whether smoking was an effect modifier of the association by including an exposure-smoking product term in the models.
In addition to age, sex, calendar year of cohort entry, and duration of follow-up on which the logistic regression was conditioned, all models were adjusted for the potential confounders measured at cohort entry based on their known association with the outcome (development of pancreatic cancer) and may possibly influence the prescribing of an ACEI or an ARB. These included excessive alcohol use, smoking status, body mass index, chronic pancreatitis, history of previous cancer (other than non-melanoma skin cancers), history of type 2 diabetes, and ever use of anti-diabetic drugs (metformin, sulfonylureas, thiazolinedediones, insulins, and others, entered individually as non-mutually exclusive variables), aspirin, non-steroidal anti-inflammatory drugs, and statins. The models were also adjusted for the ever use of diuretics, CCBs, alpha-blockers, beta-blockers, and other antihypertensive drugs, all measured between cohort entry and the year immediately before index date, and all entered as non-mutually exclusive variables.
Sensitivity analyses
We conducted three sensitivity analyses. The first consisted of repeating the primary analysis after applying longer lag periods of 2 and 3 years before index date, given uncertainties related to the latency time window (as it is not possible to delineate the timeline for the development of pancreatic cancer). Second, the primary analysis was repeated using an alternate exposure definition, consisting of receiving at least four prescriptions within a 12-month period of ACEIs or ARBs. Finally, to account for the missing information on smoking status, sensitivity analyses were conducted using multiple imputation methods.
Results
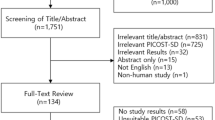
A total of 547 566 patients were included in the cohort (Figure 1). The mean (s.d.) age at entry of the cohort was 68.6 (10.4) years and 51.4% were males. The cohort generated 3 040 332 person-years of follow-up during which 866 patients were newly diagnosed with pancreatic cancer (crude incidence rate: 28.5 (95% CI: 26.6–30.4 per 100 000 person-years)).
The baseline characteristics of the cases and matched controls are listed in Table 1. Compared with matched controls, cases were more likely to have a lower body mass index, but more likely to have a history of excessive alcohol use, to have ever smoked, and to have ever used anti-diabetic drugs. Cases were more likely to have been diagnosed with type 2 diabetes than controls (10.2% vs 7.6%, respectively), whereas the prevalence of previous cancer was similar in both groups (Table 1).
Table 2 presents the results of the primary and secondary analyses for ACEIs. Overall, compared with the use of other antihypertensive drugs, the use of ACEIs was not associated with a decreased risk of pancreatic cancer (OR: 1.01, 95% CI: 0.86–1.17). In addition, there was no evidence of a duration– or dose–response relationship, with all ORs around null the value for the different secondary exposure definitions (Table 2). Smoking status did not modify the association (p-interaction=0.27; Supplementary Table 1).
Table 3 presents the results of the primary and secondary analyses for ARBs. Overall, compared with the use of other antihypertensive drugs, the use of ARBs was not associated with a decreased risk of pancreatic cancer (OR: 0.93, 95% CI: 0.75–1.15). In secondary analyses, cumulative durations of 12–36 months and receiving between 12 and 36 prescriptions were associated with 38% and 39% risk reductions, respectively (Table 3). The time since initiation of ARBs did not influence the incidence of pancreatic cancer (Table 3). Smoking did not significantly modify the association (p-interaction=0.20; Supplementary Table 1). Finally, compared with never use of any ACEI or ARB, ever use of ACEIs alone, ARBs alone, or history of both ACEIs and ARBs was not associated with a decreased incidence of pancreatic cancer (Supplementary Table 2).
Sensitivity analyses
Similar findings were observed when repeating the primary analyses using alternate lag periods (2 and 3 years) (Supplementary Table 3), and using a stricter exposure definition for ACEIs and ARBs (Supplementary Tables 4 and 5). For the latter, a decreased risk of pancreatic cancer remained in the 12–36 months cumulative duration category (Supplementary Table 5). Finally, similar findings were obtained when using multiple imputation methods for patients with missing smoking information (Supplementary Table 6).
Discussion
To our knowledge, this is the first population-based study to investigate the association between the use of ACEIs and ARBs on the incidence of pancreatic cancer. Overall, there was no association between the use of ACEIs and ARBs on this outcome when compared with patients using other antihypertensive drugs. However, in a secondary analysis, we observed a 38% decreased risk of pancreatic in patients whose ARB cumulative duration of use varied between one and three years. Overall, sensitivity analyses yielded findings consistent with those of the primary analyses.
The data related to cancer development and use of ACEIs and ARBs remains vague. Initially, there were concerns that ACEIs and ARBs may increase the risk of developing cancer, but subsequent studies did not substantiate these claims (Lever et al, 1998; Coleman et al, 2008; Bangalore et al, 2011; Collaboration, 2011; Mc Menamin et al, 2012). Furthermore, to our knowledge, no study has investigated the impact of ACEIs and ARBs on pancreatic cancer incidence specifically. One study investigated their effect on treatment outcomes in a cohort of 155 patients with pancreatic cancer receiving gemcitabine chemotherapy (Nakai et al, 2010). Overall, the use of ACEIs or ARBs was associated with an improvement in overall survival compared with hypertensive and non-hypertensive patients (15.1 months vs 8.9 months and 9.5 months, respectively), generating a 42% risk reduction compared with the group without hypertension (HR: 0.58, 95% CI: 0.34–0.95) (Nakai et al, 2010). However, this study was likely affected by immortal time bias (Suissa, 2008), which was introduced by misclassifying the time between diagnosis and the first ACEI or ARB prescription as exposed. Moreover, by design, patients necessarily had to survive to be subsequently considered exposed, otherwise they would have been considered unexposed. This bias may have greatly exaggerated the purported effects of these agents on overall survival.
From a biological standpoint, there exists a body of pre-clinical data, which seems to indicate that the RAS has an important role in carcinogenesis and that its blockade may result in a protective effect against cancer (Fujimoto et al, 2001; Arafat et al, 2007; Anandanadesan et al, 2008; Chauhan et al, 2013; Masamune et al, 2013). Several mechanisms have been proposed to explain a possible protective effect of ACEIs and ARBs. Vascular endothelial growth factor (VEGF) is a well-known factor in neoplasm formation, as well as a target for drug therapies and mediates tumour growth via its role in the formation of new blood vessels (Dvorak et al, 1995). In one study, RAS and VEGF expression in the pancreas were shown to be related and exposure of an ARB in vitro diminished the expression of VEGF and cell growth (Arafat et al, 2007; Fendrich et al, 2010). Other proposed mechanisms implicate the blockade of RAS in apoptosis; one study showing an interaction with NF-kappa b and another through pancreatic stellate cell deactivation (Amaya et al, 2004; Gong et al, 2010; Masamune et al, 2013).
There are several possibilities why our findings are generally inconsistent with the pre-clinical data. First, the doses used in experimental studies may be different from the ones used in the clinical setting. Second, the treatment of hypertension often requires multi-agent therapy (Campbell et al, 2010; McManus et al, 2012), and thus the risk of the outcome may have been influenced by the use of other antihypertensive drugs. Although our models adjusted for the use of other antihypertensive drugs, it is possible that the doses of ACEIs and ARBs were adjusted when used in combination therapy with other antihypertensive drugs. This may explain why decreased risk observed with ARBs was lost after >3 years of use, and may thus reflect, at least in part, a change in the dosing regimen. It is also possible that this analysis lacked statistical power, as it was based on 43 exposed cases generating a wide CI.
This study has several strengths. First, we assembled a large population-based cohort of patients initially treated with antihypertensive drugs, which generated a significant number of cases. Second, confounding by indication was likely minimised by restricting the cohort to patients using antihypertensive drugs. Third, the use of a nested case–control analysis with risk set sampling allowed us to assess exposure in a time-dependent fashion, thereby eliminating immortal time bias (Suissa, 2008). Finally, the use of the CPRD allowed us to adjust the models for a number of potential confounders often absent in administrative databases, such as smoking and body mass index.
This study also has some limitations. First, given the observational nature of the study, there may be some concerns regarding residual confounding owing to unmeasured covariates, such as the severity of hypertension. However, a relationship between hypertension and pancreatic cancer incidence has never been documented as there have been many conflicting reports (Stolzenberg-Solomon et al, 2002; Rosato et al, 2011; Eijgenraam et al, 2013). Second, exposure misclassification is a possibility, as the CPRD records prescriptions written by general practitioners and not specialists. Furthermore, patient compliance with the pharmacotherapy is unknown. Such misclassifications would tend to bias the point estimates toward the null. Misclassification of the outcome (pancreatic cancer) is also possible, although we do not expect this misclassification to be differential between patients exposed and unexposed to ACEIs and ARBs in this cohort of patients using antihypertensive drugs. Finally, the average cohort follow-up was 5.5 years, and thus this may be considered a short duration to observe the long-term effects of ACEIs and ARBs, although the maximal follow-up was 16 years.
In summary, this is the first large population-based study investigating the association between the use of agents acting on RAS and the incidence of pancreatic cancer in patients receiving antihypertensive drugs. Our findings suggest that, overall, there is no association between the use of ACEIs and ARBs on the incidence of pancreatic cancer in this population. However, in secondary analyses, 1–3 years of ARB use was associated with a decreased risk of pancreatic cancer. These findings will need to be replicated in studies conducted in other settings.
Change history
03 January 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Amaya K, Ohta T, Kitagawa H, Kayahara M, Takamura H, Fujimura T, Nishimura G, Shimizu K, Miwa K (2004) Angiotensin II activates MAP kinase and NF-kappaB through angiotensin II type I receptor in human pancreatic cancer cells. Int J Oncol 25: 849–856.
Anandanadesan R, Gong Q, Chipitsyna G, Witkiewicz A, Yeo CJ, Arafat HA (2008) Angiotensin II induces vascular endothelial growth factor in pancreatic cancer cells through an angiotensin II type 1 receptor and ERK1/2 signaling. J Gastrointest Surg 12: 57–66.
Arafat HA, Gong Q, Chipitsyna G, Rizvi A, Saa CT, Yeo CJ (2007) Antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll Surg 204: 996–1005;, discussion 1005-6.
Arnold SA, Rivera LB, Carbon JG, Toombs JE, Chang C, Bradshaw AD, Brekken RA (2012) Losartan slows pancreatic tumor progression and extends survival of SPARC-null mice by abrogating aberrant TGFb activation. Plos One 7: 18.
Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, Gupta AK, Sever PS, Gluud C, Messerli FH (2011) Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324 168 participants from randomised trials. Lancet Oncol 12: 65–82.
Becker AE, Hernandez YG, Frucht H, Lucas AL (2014) Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol 20: 11182–11198.
Campbell NR, Kaczorowski J, Lewanczuk RZ, Feldman R, Poirier L, Kwong MM, Lebel M, Mcalister FA, Tobe SW (2010) 2010 Canadian Hypertension Education Program (CHEP) recommendations: the scientific summary–an update of the 2010 theme and the science behind new CHEP recommendations. Can J Cardiol 26: 236–240.
Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popovic Z, Huang P, Bawendi MG, Boucher Y, Jain RK (2013) Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 4: 2516.
Coleman CI, Baker WL, Kluger J, White CM (2008) Antihypertensive medication and their impact on cancer incidence: a mixed treatment comparison meta-analysis of randomized controlled trials. J Hypertens 26: 622–629.
COLLABORATION, A. R. B. T (2011) Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens 29: 623–635.
Doi C, Egashira N, Kawabata A, Maurya DK, Ohta N, Uppalapati D, Ayuzawa R, Pickel L, Isayama Y, Troyer D, Takekoshi S, Tamura M (2010) Angiotensin II type 2 receptor signaling significantly attenuates growth of murine pancreatic carcinoma grafts in syngeneic mice. BMC Cancer 10: 67.
Dvorak HF, Brown LF, Detmar M, Dvorak AM (1995) Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 146: 1029–1039.
Eijgenraam P, Heinen MM, Verhage BA, Keulemans YC, Schouten LJ, Van Den Brandt PA (2013) Diabetes type II, other medical conditions and pancreatic cancer risk: a prospective study in The Netherlands. Br J Cancer 109: 2924–2932.
Fendrich V, Chen NM, Neef M, Waldmann J, Buchholz M, Feldmann G, Slater EP, Maitra A, Bartsch DK (2010) The angiotensin-I-converting enzyme inhibitor enalapril and aspirin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Gut 59: 630–637.
Fujimoto Y, Sasaki T, Tsuchida A, Chayama K (2001) Angiotensin II type 1 receptor expression in human pancreatic cancer and growth inhibition by angiotensin II type 1 receptor antagonist. FEBS Lett 495: 4.
Garcia Rodriguez LA, Perez Gutthann S (1998) Use of the UK general practice research database for pharmacoepidemiology. Br J Clin Pharmacol 45: 419–425.
Gong Q, Davis M, Chipitsyna G, Yeo CJ, Arafat HA (2010) Blocking angiotensin ii type 1 receptor triggers apoptotic cell death in human pancreatic cancer cells. Pancreas 39: 581–594.
Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ (2010) Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 69: 4–14.
Jick SS, Kaye JA, Vasilakis-Scaramozza C, Garcia Rodriguez LA, Ruigomez A, Meier CR, Schlienger RG, Black C, Jick H (2003) Validity of the general practice research database. Pharmacotherapy 23: 686–689.
Lam KY, Leung PS (2002) Regulation and expression of a renin-angiotensin system in human pancreas and pancreatic endocrine tumours. Eur J Endocrinol 146: 567–572.
Lawrenson R, Williams T, Farmer R (1999) Clinical information for research; the use of general practice databases. J Public Health Med 21: 299–304.
Lever AF, Hole DJ, Gillis CR, Mccallum IR, Mcinnes GT, Mackinnon PL, Meredith PA, Murray LS, Reid JL, Robertson JWK (1998) Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 352: 179–184.
Masamune A, Hamada S, Kikuta K, Takikawa T, Miura S, Nakano E, Shimosegawa T (2013) The angiotensin II type I receptor blocker olmesartan inhibits the growth of pancreatic cancer by targeting stellate cell activities in mice. Scand J Gastroenterol 48: 602–609.
Mc Menamin UC, Murray LJ, Cantwell MM, Hughes CM (2012) Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: a systematic review. Cancer Causes Control 23: 221–230.
McManus RJ, Caulfield M, Williams B (2012) NICE hypertension guideline 2011: evidence based evolution. BMJ 344: e181.
Nakai Y, Isayama H, Ijichi H, Sasaki T, Sasahira N, Hirano K, Kogure H, Kawakubo K, Yagioka H, Yashima Y, Mizuno S, Yamamoto K, Arizumi T, Togawa O, Matsubara S, Tsujino T, Tateishi K, Tada M, Omata M, Koike K (2010) Inhibition of renin-angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer 103: 1644–1648.
Noguchi M, Yoshiji H, Ikenaka Y, Namisaki T, Kitade M, Kaji K, Yoshii J, Yanase K, Yamazaki M, Tsujimoto T, Kawaratani H, Fukui H (2009) Synergistic inhibitory effect of gemcitabine and angiotensin type-1 receptor blocker, losartan, on murine pancreatic tumor growth via anti-angiogenic activities. Oncol Rep 22: 6.
Peach MJ (1977) Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 57: 313–370.
Perez-Diaz I, Guzmán C, Olivares-Reyes JA, Ramirez T, Gutierrez-Reyes G, Hiriart M, Robles-Diaz G (2011) Evidence of an intracellular angiotensin-generating systemand non-AT1, non-AT2 binding site in a human pancreatic cell line. Pancreas 40: 701–707.
Rosato V, Tavani A, Bosetti C, Pelucchi C, Talamini R, Polesel J, Serraino D, Negri E, La Vecchia C (2011) Metabolic syndrome and pancreatic cancer risk: a case-control study in Italy and meta-analysis. Metabolism 60: 1372–1378.
Stolzenberg-Solomon RZ, Pietinen P, Taylor PR, Virtamo J, Albanes D (2002) A prospective study of medical conditions, anthropometry, physical activity, and pancreatic cancer in male smokers (Finland). Cancer Causes Control 13: 417–426.
Suissa S (2008) Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 167: 492–499.
Acknowledgements
This study was funded, in part, by grants from the Canadian Institutes of Health Research, and the Arnie Vered Fund Against Cancer. Dr Laurent Azoulay is the recipient of a Chercheur-Boursier Career Award from the Fonds de recherche du Québec - Santé.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Mandilaras, V., Bouganim, N., Yin, H. et al. The use of drugs acting on the renin–angiotensin system and the incidence of pancreatic cancer. Br J Cancer 116, 103–108 (2017). https://doi.org/10.1038/bjc.2016.375
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.375
Keywords
This article is cited by
-
Antihypertensive drugs and pancreatic cancer risk in patients with chronic pancreatitis: a Danish nationwide population-based cohort study
British Journal of Cancer (2019)
-
Pancreatic adenocarcinoma response to chemotherapy enhanced with non-invasive radio frequency evaluated via an integrated experimental/computational approach
Scientific Reports (2017)