Abstract
Background:
The delay between onset of macroscopic haematuria and diagnosis of bladder cancer is often long.
Methods:
We evaluated timely diagnosis and health-care costs for patients with macroscopic haematuria given fast-track access to diagnostics. During a 15-month period, a telephone hotline for fast-track diagnostics was provided in nine Swedish municipalities for patients aged ⩾50 years with macroscopic haematuria. The control group comprised 101 patients diagnosed with bladder cancer in the same catchment area with macroscopic haematuria who underwent regular diagnostic process.
Results:
In all 275 patients who called ‘the Red Phone’ hotline were investigated, and 47 of them (17%) were diagnosed with cancer and 36 of those had bladder cancer. Median time from patient-reported haematuria to diagnosis was 29 (interquartile range (IQR) 14−104) days and 50 (IQR 27−165) days in the intervention and the control group, respectively (P=0.03). The median health-care costs were lower in the intervention group (655 (IQR 655−655) EUR) than in the control group (767 (IQR 490−1096) EUR) (P=0.002).
Conclusions:
Direct access to urologic expertise and fast-track diagnostics is motivated for patients with macroscopic haematuria to reduce diagnostic intervals and lower health-care expenditures.
Similar content being viewed by others
Main
Bladder cancer is diagnosed in 120 000 individuals annually in Europe, and the incidence of this disease is increasing in many countries, including Sweden (http://www.cancercentrum.se/globalassets/cancerdiagnoser/urinvagar/urinblase--och-urinrorscancer/rapport2014final.pdf). Macroscopic haematuria is the isolated alarm symptom with the highest positive predictive value for cancer (Shapley et al, 2010), with cancer detection rates of up to 24−34% depending on gender and age distribution in the investigated populations (Sultana et al, 1996; Boman et al, 2001). The total delay from initial symptom to treatment is longer than for any other tumour type (Hansen et al, 2011), and there is substantial evidence that such delay decreases disease-specific survival (Hollenbeck et al, 2010; Bourgade et al, 2014), which is not always considered by health-care providers (Hamilton et al, 2015). Owing to frequent recurrences and risk of progression of non-muscle-invasive disease and often a need for lifelong surveillance, the lifetime per-patient treatment costs are higher for bladder cancer than for all other forms of cancer (Santos et al, 2015). We therefore conducted a prospective study to investigate fast-track hotline (the ‘Red Phone’) to receiving specialised urologic care for patients with macroscopic haematuria evaluating lead times, patient-reported experiences (PREM), tumour characteristics, and health-care costs.
Materials and methods
The primary catchment area of Skåne University Hospital has 625 000 inhabitants. Herein, the Red Phone hotline to a clinical nurse working at the Department of Urology was introduced for patients aged ⩾50 years with macroscopic haematuria. The Red Phone was open 0800−1200 hours Monday to Friday. The study encompassed nine municipalities and covered the period 17 March 2014 to 17 June 2015. Exclusion criteria were a previous bladder cancer diagnosis with regular follow-ups, evaluation for haematuria within one year, or transurethral surgery within two months. The nurse consultant scheduled a patient for S-creatinine testing, voided urinary cytology, an appointment with a urologist for flexible cystoscopy with the same priority as other patients referred for macroscopic haematuria in the department, and CT urography within 2 weeks. Thus, the intervention gave access for patients with macroscopic haematuria that fulfilled the inclusion criteria to bypass regular referral systems.
The control group comprised all other patients in the same nine municipalities who had at least one episode of macroscopic haematuria and were diagnosed with bladder cancer during the study period at the Department of Urology, Skåne University Hospital after regular referral, that is, all patients outside the intervention group; these patients were retrospectively identified through the Swedish National Bladder Cancer Registry. The Swedish National Bladder Cancer Registry registers all patients with bladder cancer since 1997 including address, TNM-stage, size, number of tumours and primary treatment (http://www.cancercentrum.se/globalassets/cancerdiagnoser/urinvagar/urinblase--och-urinrorscancer/rapport2014final.pdf). Data on educational level were obtained from both PREM questionnaires (http://www.cancercentrum.se/globalassets/vara-uppdrag/kunskapsstyrning/kvalitetsregister/sydost/patientenkater/prem_projektrapport_sydost_-nov_2013.pdf) and telephone interviews. The Regional Ethical Review Board in Lund approved the study (Dnr 2013/457 and 2015/154).
Before initiating this project, information about the study was given directly to representatives from the primary care centres and was displayed on the home pages of the Department of Urology, Skåne University Hospital, and Region Skåne Primary Care. Furthermore, the general public got access to the ‘Red Phone’ hotline via the home page of the Department of Urology but chiefly through the national web-based health information-system platform (www.1177.se). 1177 is a nationwide health-care guide with a 24-h open phone service where 1500 specially trained nurses are employed to provide general health advice and to guide patients to appropriate health care. General information as well as regional information, for example, the present study, is available on the internet-based information-system platform. During the study period, the 1177 phone service and website instructed all patients who fulfilled the inclusion criteria to call the ‘Red Phone’ hotline, diverting these patients away from their primary health-care centres.
To ascertain dates of haematuria, patients alive at the end of the study in the intervention and the control group were interviewed by a research nurse (AL); such dates were also retrieved from patient charts at all levels (primary care and hospital) and discrepancies between dates were solved after a consensus discussion during the telephone interview. In the intervention group, date of haematuria was also ascertained when the patient called the Red Phone hotline. Furthermore, the following information was obtained from the Swedish National Bladder Cancer Registry; dates of referral to urologist, diagnosis, first visit to urologist, and transurethral resection; clinical tumour stage and grade; number of tumours (1, 2, 3, 4, 5, 6−10 or >10); largest tumour diameter (0−10, 11−30 or >30 mm). Date of diagnosis was defined as the earliest time point of tumour identification (at cystoscopy or CT urography).
To assess PREM, a validated PREM questionnaire (http://www.cancercentrum.se/globalassets/vara-uppdrag/kunskapsstyrning/kvalitetsregister/sydost/patientenkater/prem_projektrapport_sydost_-nov_2013.pdf) was distributed to patients in both groups with a bladder cancer diagnosis 3−12 months after diagnosis (n=36 and 101). Responses could be provided online or in writing using a preaddressed envelope, and a reminder was sent after 2 weeks.
Data on resource use for outpatient care (including primary care), inpatient care, and medications related to bladder cancer over the period from macroscopic haematuria to diagnosis of bladder cancer were obtained from patient charts at primary health-care centres and hospitals, and through telephone interviews with patients. Unit costs of outpatient care, inpatient care, and medications were acquired from regional and national price lists in Sweden (http://www.skane.se/upload/webbplatser/sodra%20regionvardsnamnden/prislista/2015/helaprislistan2015.pdf; http://www.fass.se; http://webbutik.skl.se/bilder/artiklar/pdf/7164-395-7.pdf; according to regional and national price lists for 2015). Also costs for patients’ calling the ‘Red Phone’ was accounted for, however indirect costs were not estimated. When comparing costs between the control and intervention group, the analysis was restricted to bladder cancer due to the few numbers of other cancers diagnosed in the intervention group. Furthermore, due to lack of a validated control group for patients with negative haematuria investigations, cost calculations were not performed for patients without bladder cancer diagnosis.
Differences between the control and the intervention group were investigated using the Wilcoxon’s rank-sum test, and P values <0.05 were considered significant. Fischer’s exact test was used for categorical data.
Results
During the study period, 281 eligible individuals called the ’Red Phone’. Six declined further examinations, leaving 275 for evaluation (Figure 1). Of these, 123 (45%) had normal findings, 47 (17%) were diagnosed with a cancer (36 of those with bladder cancers), and 105 (38%) were diagnosed with benign conditions causing the haematuria (Table 1A). The control group comprised the 101 patients in the nine municipalities who were diagnosed with bladder cancer during the same period but not within our study. The median age (interquartile range (IQR)) was 72 (64−77) year in the intervention group and 74 (67−80) year in the control group (P=0.37). Gender distribution differed: 17/36 (47%) females in the intervention group compared with 18/101 (18%) in the control group (P=0.001).
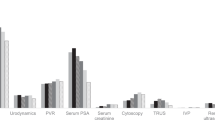
Distribution of bladder cancer stages and grades was similar in the two groups (Table 1B), and this also applied when stage and grade were stratified into low-risk non-muscle-invasive bladder cancer (NMIBC; TaG1−TaG2), high-risk NMIBC, and muscle-invasive/metastatic disease (P=0.14). The distribution of the number of tumours (stratified as 1, 2, 3, 4, 5, 6−10, and >10) was also similar in the two groups (data not shown), whereas tumour size differed, with significantly larger tumours in the control group (P=0.04) (Table 1B). Median time from patient-reported macroscopic haematuria to diagnosis was 29 (IQR 14−104) days in the intervention group but 50 (IQR 27−165) days in the control group (P=0.03). Median time between date of referral to diagnosis was also significantly shorter in the intervention group (8 days (IQR 2−13)) than in the control group (19 days (IQR 5−32)) (P=0.003), whereas the median number of days from macroscopic haematuria to referral was similar in the two groups (14 (IQR 3−98) and (33 (IQR 5−149), respectively) (P=0.32). The other lead times from macroscopic haematuria are presented in Figure 2 and Table 2.
PREM data were obtained from 17/36 (47%) and 80/101 (79%) patients in the intervention and the control group, respectively (P=0.002). Levels of education were comparable in the two groups (intervention vs control subjects): 11% and 24% primary school, 56% and 35% high school, and 33% and 41% university (P=0.26). The 46 questions on the PREM-instrument were designed to investigate patient perceptions of dimensions related to: lead times, diagnosis, contact and coordination of health care, information, rehabilitation, and support. No differences were found between the groups for any of the 46 questions or the sum of responses for each dimension measured (data not shown).
The median total care cost per patient (including inpatient care, outpatient care, and medication) was 655 (IQR 655−655) EUR in the intervention group compared with 767 (IQR 490−1096) EUR in the control group (P=0.002; Table 3A). The higher median total care costs in the control group was driven by a higher median number of health-care contacts in the control group (three (IQR two−four)) than in the intervention group (two (IQR two−two)) (P=0.01). The distribution of contacts with different health-care providers for the two groups is illustrated in Figure 3. The intervention group had only been in contact with the urology department and the red phone at the same department, with the exception of one patient who also had one visit to a primary health-care centre. The control group had been in contact with several different health-care providers, of which most frequent were primary health care (45% of the contacts), urology department (29% of the contacts) and emergency ward (13% of the contacts). The proportion of patients receiving the most frequent interventions in the intervention and control group is presented in Table 3B. Inpatient care was given only to subjects in the control group (17 patients), but these patients were few in number and hence not reflected in the inpatient care median costs (Table 3A). Likewise, few patients received medication, and thus the median cost of medication was actually 0 SEK in both groups. Medical prescriptions were made twice in the intervention group and on 57 in the control group. The most commonly prescribed pharmaceuticals in the latter group were ciprofloxacin (12 prescriptions), pivmecillinam (11 prescriptions) and nitrofurantoin (7 prescriptions).
Discussion
The positive predictive value (PPV) for macroscopic haematuria and cancer was 17% (47/275) in the intervention group in the present study, but was 10.3% in an unselected population with macroscopic haematuria (Bruyninckx et al, 2003). The higher value noted in our investigation is probably related to the age criteria applied (only patients aged ⩾50 year included), because the PPV increases with age (Schmidt-Hansen et al, 2015). In countries with a gatekeeper system where access to specialist care requires referral from primary care, a recent case-control study reported an estimated PPV for bladder cancer of 6.1% in patients aged ⩾60 years (Shepard et al, 2012), indicating that community-based studies might have lower PPV. However, that study did not account for upper urinary tract cancer and depended on physicians correctly classifying macroscopic vs microscopic haematuria. In Sweden asymptomatic non-visible haematuria is no longer investigated for urologic cancer due to a low diagnostic yield (Holmäng, 2016).
Reduced median diagnostic delay from macroscopic haematuria to established bladder cancer (29 days in the intervention group vs 50 days in the control group) was attributed to the accelerated investigation of macroscopic haematuria, as the median time from macroscopic haematuria to referral was similar in the intervention and control groups (14 and 33 days, respectively; P=0.32). The diagnostic delay in both groups seems favourable, considering that a median of 134 days was recently found in Denmark (Hansen et al, 2011). However the Danish study also included patients diagnosed with bladder cancer based on with other symptoms in addition to macroscopic haematuria, which might have contributed to the longer lead times in that investigation, because urgency is associated with longer delay compared with haematuria (Månsson et al, 1993). Despite equal priority to patients in the control group and intervention group, the explanations for shorter diagnostic delay in the intervention group are due to multiple causes. For example, no time-loss for referral letters from the primary care physician to reach the department of urology has contributed to the shorter diagnostic delay in the intervention group. Higher priority for CT-urographies when booked through department of urology immediately when the patient called the ‘Red Phone’, as compared with regular referral with booking from either the primary care physician or when referral letter reached the department of urology. Also frequently patients in the control group were prescribed antibiotics by the primary health-care physician and first after re-evaluation referred for haematuria investigation might have contributed to shorter diagnostic delay in the intervention group, even though the difference between days from macroscopic haematuria to referral were not statistically different between the two groups (14 (IQR 3−98) and (33 (IQR 5−149), intervention and control group, respectively) (P=0.32).
Increasing age (Liedberg et al, 2015) and female gender (Dobruch et al, 2016) are both associated with higher tumour stage at diagnosis. Our finding that tumours in the control group were significantly larger despite similar median age in the two groups and females being overrepresented in the intervention group (47 vs 18% in the control group) is difficult to explain (Table 1B). Perhaps this observation can be attributed to the longer diagnostic delay in the control group. The suggestion that larger tumours grow more rapidly than smaller (Gofrit et al, 2006), also supports the conclusion that the larger tumour size observed in our control group is biologically relevant and related to the longer diagnostic delay per se, considering that the difference was noted only for tumours with a maximum diameter of >30 mm. However, the differences in tumour sizes observed may also have several other causes, that is, selection bias linked to more health-conscious individuals in the intervention group and other unknown differences in risk factors between the groups as, for example, smoking-status, although educational levels and age were comparable.
Females diagnosed with bladder cancer were overrepresented in the intervention group compared with the control group (47 vs 18%). The cause for this overrepresentation is unknown, though females have in several studies shown higher health awareness, potentially linked to experiences from screening programmes for breast cancer and cervical cancer. A longer diagnostic delay for women than men has previously been explained by impeded referral to a urologist (Garg et al, 2014), and a Swedish study also demonstrated that women more often than men were referred a second or a third time before being diagnosed with bladder cancer (26 vs 9%) (13). However, we did not find any gender differences between time from haematuria to diagnosis in the present study (P=0.47, data not shown). Thus it seems that direct access to urologic care (e.g., via a hotline like ‘the Red Phone’) for individuals with macroscopic haematuria is especially important for women as it may counteract gender-related disparity (Månsson et al, 1993; Garg et al, 2014). Direct urologic referral is indicated also for females, as well as for men, with culture positive urinary tract infection, as the risk for malignancy in a prospective fast-track haematuria study was equally high for culture negative and culture positive patients, (20 and 24%, respectively) (Vasdev and Thorpe, 2013).
Mass media public health campaigns such as ‘Blood in Pee’ launched by Public Health England (Hughes-Hallett et al, 2015) may represent an alternative or complementary strategy to accelerated haematuria evaluation, and intuitively would also reduce the longer diagnostic delay reported for women (Garg et al, 2014) and patients with urinary tract infections and haematuria that are later diagnosed with bladder cancer (Richards et al, 2015). There was no rise in bladder cancer diagnoses during the English campaign, but that does not exclude that such an intervention results in shorter lead times for bladder cancer diagnosis, because public awareness of the severity of macroscopic haematuria and the high risk of malignancy is probably limited. Haematuria public awareness campaigns can also be favoured by emphasising the preventive effect of smoking cessation, as indicated by a study showing that only one in four patients in a urologic outpatient unit recognised that smoking as a risk factor for bladder cancer (Bjurlin et al, 2012).
A similar type of expedited cancer diagnostics is now launched throughout the Nordic countries based on a Danish model which has been demonstrated to decrease lead times and improve timely access to cancer care (http://skl.se/halsasjukvard/kunskapsstodvardochbehandling/cancervard/overenskommelsecancervard.2049.html). Initiatives such as the Red Phone aiming at standardised care pathways or fast-track access to urologic care may be particularly relevant in diagnoses where system delays have a larger impact than patient delays. A median patient delay of 5 days has been reported in patients with macroscopic haematuria, whereas a median delay in care attributed to by doctor’s (or health-care facilities) was 53 days (Månsson et al, 1993), which suggests that interventions should focus on the primary care delay in cancer referral (Mansell et al, 2011).
Health-care costs calculated for the period from macroscopic haematuria to bladder cancer diagnosis were lower in the intervention group (Table 3A), which was largely explained by a lower number of health-care contacts in the intervention group. In the intervention group only one patient visited a primary health-care centre. Thus, patients with macroscopic haematuria without other symptoms necessitating acute health-care contact, such as fever due to haemorrhagic cystitis or clot retention, can for the sake of time and costs be spared visits to other health-care givers. The higher costs in the control group may also be attributed to larger tumours in the control group, which is suggested by excess costs for inpatient care in the later group. Emergency presentation has been reported in 18.5% of bladder cancer patients in the United Kingdom between 2006 and 2010 (Abel et al, 2015). Thus, health-care costs in Sweden could probably be reduced if all individuals with macroscopic haematuria could immediately call ‘the Red Phone’ and undergo expeditious investigation in an outpatient setting, instead of consuming additional health-care contacts, inpatient or emergency care. Also, larger tumours are presumably more costly to treat due to more frequent need for re-resections, adjuvant instillations, and radical surgery, but these costs were not considered in the present analysis. Furthermore, the reduced need for a diagnostic cystoscopy in 17% of patients with obvious bladder cancer on a CT urography (Blick et al, 2012), or an even higher proportion with an optimally designed CT urography including a corticomedullary phase (Helenius et al, 2016), was not fully exploited in the current study to reduce costs as 94% of the patients in both groups were submitted to a cystoscopy (Table 3B).
The limitations of our investigation are related primarily to differences in selection mechanisms between the two study groups and the relatively limited number of patients included. Age and tumour stage distribution were comparable, but sex and tumour size differed, which may have influenced the results. Moreover, 17 patients in the control group were hospitalised due to macroscopic haematuria, and it is not known how many of those hospitalisations that could have been prevented by the fast-track access to urologic care that was as offered to the intervention group. Also, the present results are relevant for countries with diagnostic delays in cancer care, although the impact on health-care costs may vary between different care systems, thus external validation of the ‘Red phone’ hotline is needed. Furthermore, the proportion of patients who received information about the project from the home page of the Department of Urology, the web-based health information-system platform at www.1177.se or by other means is not known. Also, potential costs for patients with macroscopic haematuria during the study period that never were investigated before the current study was closed that later will appear with a bladder cancer diagnosis, were not possible to take into account.
Conclusions
Compared with the regular referral, the Red Phone hotline intervention for accelerated investigation of patients with macroscopic haematuria via direct access to urologic care decreased lead times, fewer health-care contacts and reduced health-care costs for patients diagnosed with bladder cancer.
Change history
27 September 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abel GA, Shelton J, Johnson S, Elliss-Brookes L, Lyratzopoulos G (2015) Cancer-specific variation in emergency presentation by sex, age and deprivation across 27 common and rarer cancers. Br J Cancer 112 Suppl 1: S129–S136.
Bjurlin MA, Cohn MR, Freeman VL, Lombardo LM, Hurley SD, Hollowell CM (2012) Ethnicity and smoking status are associated with awareness of smoking related genitourinary diseases. J Urol 188: 724–728.
Blick CG, Nazir SA, Mallett S, Turney BW, Onwu NN, Roberts IS, Crew JP, Cowan NC (2012) Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic. BJU Int 110: 84–94.
Boman H, Hedelin H, Holmäng S (2001) The results of routine evaluation of adult patients with haematuria analysed according to referral form information with 2-year follow-up. Scand J Urol Nephrol 35: 497–501.
Bourgade V, Drouin SJ, Yates DR, Parra J, Bitker MO, Cussenot O, Rouprêt M (2014) Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J Urol 32: 475–479.
Bruyninckx R, Buntinx F, Aertgeerts B, Van Casteren V (2003) The diagnostic value of macroscopic haematuria for the diagnosis of urological cancer in general practice. Br J Gen Pract 53: 31–35.
Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA (2016) Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol 69 (2): 300–310.
Garg T, Pinheiro LC, Atoria CL, Donat SM, Weissman JS, Herr HW, Elkin EB (2014) Gender disparities in hematuria evaluation and bladder cancer diagnosis: a population based analysis. J Urol 192: 1072–1077.
Gofrit ON, Pode D, Lazar A, Katz R, Shapiro A (2006) Watchful waiting policy in recurrent Ta G1 bladder tumors. Eur Urol 49: 303–306.
Hamilton W, Stapley S, Campbell C, Lyratzopoulos G, Rubin G, Neal D (2015) For which cancers might patients benefit most from expedited symptomatic diagnosis? Construction of a ranking order by a modified Delphi technique. BMC Cancer 15: 820–828.
Hansen RP, Vedsted P, Sokolowski I, Sondergaard J, Olesen F (2011) Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv Res 11: 284–295.
Helenius M, Dahlman P, Lonnemark M, Brekkan E, Wernroth L, Magnusson A (2016) Comparison of post contrast CT urography phases in bladder cancer detection. Eur Radiol 26: 585–591.
Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Skolarus TA, Kim SP, Montie JE, Lee CT, Wood DP Jr, Miller DC (2010) Delays in diagnosis and bladder cancer mortality. Cancer 116: 5235–5242.
Holmäng S (2016) Mikroskopisk hematuri – ingen varningsklocka för cancer i urinvägarna. Läkartidningen 113: 1–3.
Hughes-Hallett A, Browne D, Mensah E, Vale J, Mayer E (2015) Assessing the impact of mass media public health campaigns. 'Be Clear on Cancer ‘blood in pee’: a case in point. BJU Int 117: 570–575.
Liedberg F, Hagberg O, Holmäng S, Hosseini Aliabad A, Jancke G, Ljungberg B, Malmström PU, Åberg H, Jahnson S (2015) Local recurrence and progression of non-muscle-invasive bladder cancer in Sweden: a population-based follow-up study. Scand J Urol 49: 290–295.
Mansell G, Shapley M, Jordan JL, Jordan K (2011) Interventions to reduce primary care delay in cancer referral. Br J Gen Pract 61: e821–e835.
Månsson A, Anderson H, Colleen S (1993) Time lag to diagnosis of bladder cancer-influence of psychosocial parameters and level of health-care provision. Scand J Urol Nephrol 27: 363–369.
Richards KA, Ham S, Cohn JA, Steinberg GD (2015) Urinary tract infection-like symptom is associated with worse bladder cancer outcomes in the Medicare population: Implications for sex disparities. Int J Urol 23: 42–47.
Santos F, Dragomir A, Zakaria AS, Kassouf W, Aprikian A (2015) Health-care services utilization and costs associated with radical cystectomy for bladder cancer: a descriptive population-based study in the province of Quebec, Canada. BMC Health Serv Res 15: 308.
Schmidt-Hansen M, Berendse S, Hamilton W (2015) The association between symptoms and bladder or renal tract cancer in primary care: a systematic review. Br J Gen Pract 65: e769–e775.
Shapley M, Mansell G, Jordan JL, Jordan KP . Positive predictive values of ≥5% in primary care for cancer: systematic review (2010) Br J Gen Pract 60: e366–e377.
Shepard EA, Stapley S, Neal RD, Rose P, Walter FM, Hamilton WT (2012) Clinical features of bladder cancer in primary care. Br J Gen Pract 62: e598–e604.
Sultana SR, Goodman CM, Byrne DJ, Baxby K (1996) Microscopic haematuria: urological investigation using a standard protocol. Br J Urol 78: 691–696.
Vasdev N, Thorpe AC (2013) Should the prescence of culture positive urinary tract infection exclude patients from rapid evaluation hematuria protocols? Urol Oncol 31: 909–913.
Acknowledgements
This research was supported by grants from The Swedish Cancer Society (CAN 2014/448) and Lund Medical Faculty (ALF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Liedberg, F., Gerdtham, U., Gralén, K. et al. Fast-track access to urologic care for patients with macroscopic haematuria is efficient and cost-effective: results from a prospective intervention study. Br J Cancer 115, 770–775 (2016). https://doi.org/10.1038/bjc.2016.265
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.265
Keywords
This article is cited by
-
Symptoms and diagnostic delays in bladder cancer with high risk of recurrence: results from a prospective FinnBladder 9 trial
World Journal of Urology (2020)
-
Bladder cancer
Nature Reviews Disease Primers (2017)