Abstract
Background
The American Urological Association makes recommendations for evaluation and testing for lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH) to help primary care providers and specialists identify LUTS/BPH and harmful related conditions including urinary retention and prostate or bladder cancer. Our understanding of provider adherence to these Guidelines is limited to single-site or nonrepresentative settings.
Methods
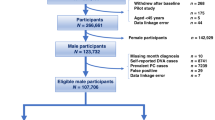
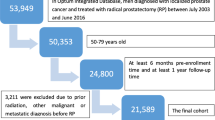
We analyzed two insurance claims databases: the Optum® de-identified Clinformatics® Data Mart database for privately insured males aged 40–64 years (N ≈ 1,650,900 annually) and the Medicare 5% Sample for males aged ≥65 years (N ≈ 546,000 annually). We calculated the annual prevalence of LUTS/BPH and comorbid bladder cancer and bladder stones from 2004 to 2013. We additionally examined LUTS/BPH incidence and adherence to testing guidelines in a cohort of men newly diagnosed with LUTS/BPH in 2009.
Results
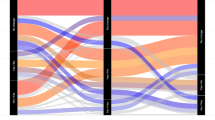
While LUTS/BPH prevalence and incidence increased with increasing age, evaluation testing became less common. Urinalysis was the most common testing type but was performed in <60% of incident patients. Serum prostate-specific antigen (PSA) was the second most common test across age groups (range: 15–34%). Prevalence of comorbid bladder cancer (range: 0–4%), but not bladder stones (range: 1–2%), increased with increasing age.
Conclusions
Although older men were at greater risk of LUTS/BPH than younger men, they were less likely to undergo testing at diagnosis. Recommended testing with urinalysis was poor despite higher prevalence of bladder cancer in older men and a standard recommendation for urinalysis since 1994. Providers should be more cognizant of AUA Guidelines when assessing LUTS/BPH patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
These analyses were conducted as part of the Urologic Diseases in America annual research report, which appear online at https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/urologic-diseases-in-america.
Material availability
All material appearing in this report is in the public domain and may be reproduced or copied without permission.
References
Auffenberg GB, Gonzalez CM, Wolf JS Jr, Clemens JQ, Meeks W, McVary KT. An observational analysis of provider adherence to AUA guidelines on the management of benign prostatic hyperplasia. J Urol. 2014;192:1483–8.
Wei JT, Miner MM, Steers WD, Rosen RC, Seftel AD, Pasta DJ, et al. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol. 2011;186:971–6.
Welliver C, Feinstein L, Ward JB, Fwu CW, Kirkali Z, Bavendam T, et al. Trends in Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia, 2004-2013: The Urologic Diseases in America Project. J Urol. 2019. https://doi.org/10.1097/JU0000000000000499.
United States Renal Data System. USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. p. 2018.
A. U. A. Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendations. J Urol. 2003;170:530–47.
Djavan B, Fong YK, Harik M, Milani S, Reissigl A, Chaudry A, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology. 2004;64:1144–8.
McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–98.
Roehrborn CG, Boyle P, Bergner D, Gray T, Gittelman M, Shown T, et al. Serum prostate-specific antigen and prostate volume predict long-term changes in symptoms and flow rate: results of a four-year, randomized trial comparing finasteride versus placebo. PLESS Study Group. Urology. 1999;54:662–9.
Roehrborn CG, McConnell J, Bonilla J, Rosenblatt S, Hudson PB, Malek GH, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol. 2000;163:13–20.
Foster HE, Barry MJ, Dahm P, Gandhi MC, Kaplan SA, Kohler TS, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200:612–9.
Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200.
Schultzel M, Saltzstein SL, Downs TM, Shimasaki S, Sanders C, Sadler GR. Late age (85 years or older) peak incidence of bladder cancer. J Urol. 2008;179:1302–5.
Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190:419–26.
U. S. Preventive Services Task Force, Grossman DC, Curry SJ, Owens DK, Bibbins-Domingo K, Caughey AB, et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901–13.
U. S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–91.
Doolin J, Reese ZA, Cooperman JL, Mukamal KJ. Correction to: National trends in the use of PSA, urinalysis, and digital rectal exam for evaluation of lower urinary tract symptoms in men. World J Urol. 2021;39:861.
Acknowledgements
The Urologic Diseases in America project was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through a contract to Social & Scientific Systems (HHSN276201500204U). JW, EM, and LF are employed by Social & Scientific Systems, and BM of Johns Hopkins University has a subcontract with the company.
Funding
The Urologic Diseases in America project was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through a contract to Social & Scientific Systems (HHSN276201500204U).
Author information
Authors and Affiliations
Contributions
Conceived and/or designed the work that led to the submission: CW, LF, JBW, ZK, BRM, and KM. Acquired data: LF and JBW. Played an important role in interpreting the results: CW, LF, JBW, EEM, and KM. Drafted the paper: CW, LF, JBW, EEM, and KM. Revised the paper: all authors. Approved the final version: all authors. Agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Copernicus Group Independent Review Board® designated this project exempt from review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Welliver, C., Feinstein, L., Ward, J.B. et al. Poor clinical guideline adherence and inappropriate testing for incident lower urinary tract symptoms associated with benign prostatic hyperplasia. Prostate Cancer Prostatic Dis 25, 269–273 (2022). https://doi.org/10.1038/s41391-021-00435-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-021-00435-z
This article is cited by
-
When you say “Prostate”, don’t forget to say “Bladder”!
Prostate Cancer and Prostatic Diseases (2024)
-
Yoga, benign prostatic hyperplasia and lower urinary tract symptoms: a new path for clinical trials
Prostate Cancer and Prostatic Diseases (2024)
-
From BPH to male LUTS: a 20-year journey of the EAU guidelines
Prostate Cancer and Prostatic Diseases (2024)
-
Prevalence of lower urinary tract symptoms in taxi drivers: a cross-sectional web-based survey
Prostate Cancer and Prostatic Diseases (2023)