Abstract
Background:
There is an ongoing debate about the relationship between breast implants and secondary malignancies.
Methods:
Breast cancer patients undergoing surgical reconstruction after mastectomy by either implants or autologous flap were identified in the Surveillance, Epidemiology and End Results registry between 1998 and 2002. The occurrence of secondary malignancies at least 1 year after diagnosis was compared between breast reconstruction with implants vs autologous flap.
Results:
Of 7955 women, 3727 underwent reconstruction using implants and 4228 using autologous flap. The incidence of secondary tumours was similar in both the groups (hazards ratio (HR)=1.02, 95% confidence interval (CI): 0.82–1.26, P=0.880). For lung cancer, a significantly increased risk for implants (HR=2.51, 95% CI: 1.28–4.95, P=0.005) was observed.
Conclusions:
Except for lung cancer, no association between implants and secondary malignancies including lymphomas was observed.
Similar content being viewed by others
Main
Surgical breast reconstruction is an important option to improve the quality of life in women undergoing mastectomy for breast cancer. Options for breast reconstruction include tissue expander/implants or autologous reconstruction using tissue flaps. Tissue expander/implant reconstruction is the most commonly practiced alloplastic reconstructive procedure in the United States (Alderman et al, 2011) and is used as an alternative to autologous reconstruction (Lin et al, 2001; Chawla et al, 2002). Breast implants are associated with a slightly higher risk of reconstructive failure or surgical-site infection as compared with autologous reconstruction, but with lower rates of skin or flap necrosis (Tsoi et al, 2014). Recently, anaplastic large-cell lymphoma (ALCL) has been associated with reconstructive breast implants following breast cancer (Duvic et al, 1995; Keech and Creech, 1997; Agarwal et al, 2010; Jewell et al, 2011; Taylor et al, 2013), resulting in a white paper issued by the US Food and Drug Administration in 2011, based on 34 cases of breast implant-associated ALCL in an estimated 5–10 million women with breast implants (Center for Devices and Radiological Health, 2011). We assessed the potential association between secondary malignancies and the type of breast reconstruction in a large, unselected group of breast cancer patients by applying stratified propensity score matching to correct for potential case selection bias.
Materials and Methods
Database and cohort definition
The 2014 submission of the Surveillance, Epidemiology and End Results (SEER) program was used as data source. From 262 445 female breast cancer patients diagnosed between 1998 and 2002, 8044 were eligible for the analysis after exclusion of patients with in situ carcinoma (N=47 121), lacking diagnosis by histology (N=4960), secondary malignancies prior to breast cancer (N=31 489), other histology than adenocarcinoma, cystic, mucinous, serous, ductal, lobular or mixed ductal and lobular carcinoma (N=4748), other than stage I–III (N=18 358), pre- or intraoperative radiation (N=1183), lacking income data on the county level (N=1606), no subcutaneous, simple, radical or modified radical mastectomy (N=86 317), no reconstruction (N=58 619) and follow-up of <1 year (N=89). The remaining 7955 patients were grouped according to whether they had received breast reconstruction by autologous flaps or by implants.
Statistical analysis
Statistical analysis was performed using the R statistical software (www.r-project.org). After descriptive analysis, logistic regression was performed to assess the association between patient and treatment characteristics. Potential confounders were tumour stage, histology, grading, ER and PR status, type of mastectomy, local radiotherapy, year of initial diagnosis, patient age, ethnicity, marital status and census tract level of household income. Secondary malignancies were treated as time-to-event-data and counted only if they occurred at least 1 year after breast cancer diagnosis. Only the first case of breast cancer was considered to avoid the inclusion of relapses in the analysis. Secondary malignancies were grouped according to the Collaborative Stage scheme. The association between breast reconstruction and patient characteristics was analysed by multivariable logistic regression. The association between secondary malignancies and breast reconstruction by autologous flap vs implants was assessed by Cox regression stratified for age and by propensity score matching using the ‘MatchIt’ and ‘optmatch’ R packages (Ho et al, 2007). Based on the results of the matching procedure, a second Cox regression analysis was performed. Both stratified and propensity score-matched Cox regression was repeated for each entity of secondary malignancies. Finally, we assessed potential differences in smoking-related causes of death between the two study groups using Cox regression analysis.
Results
Patient characteristics
No significant trend in the annual rate of breast reconstruction on all mastectomies was found with rates of 11.0, 12.6, 12.3, 12.0 and 12.1% from 1998 to 2002 (PTrend=0.233). Of the 7955 women included in the study, 3727 (46.9%) received breast reconstruction using implants and 4228 (53.1%) received breast reconstruction using an autologous flap (Table 1). The median follow-up was 10.3 years (Interquartilerange: 9.2–11.6 years).
Secondary malignancies
A total of 874 secondary malignancies were encountered. Of these, 514 secondary breast carcinomas and 29 malignancies occurring within 1 year after breast cancer diagnosis were excluded. The 340 secondary malignancies in the analysis were distributed as follows: Lung carcinoma (N=40, 0.5%), colorectal cancer (N=38, 0.5%), endometrial cancer (N=32, 0.4%), melanoma (N=31, 0.4%), thyroid cancer (N=30, 0.4%), ovarian cancer (N=28, 0.4%), kidney cancer (N=25, 0.3%), lymphoma (N=21, 0.3%), haematological malignancies (N=21, 0.3%), bladder cancer (N=13, 0.2%), pancreatic cancer (N=12, 0.2%), anal cancer (N=8, 0.1%), neuroendocrine tumours (N=6, 0.1%), brain cancer (N=5, 0.1%), cancer of cervix uteri (N=5, 0.1%), peritoneal cancer (N=5, 0.1%), soft tissue sarcoma (N=5, 0.1%), hepatobiliary cancer (N=3), appendiceal cancer (N=2), oesophageal cancer (N=2), myeloma (N=2), parotideal cancer (N=2), bone cancer (N=1), skin cancer other than melanoma (N=1), small intestinal cancer (N=1) and cancer of the vulva (N=1).
Association between the type of breast reconstruction and secondary malignancies
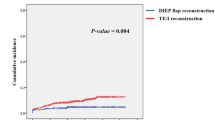
In the flap and implant group, 176 (4.2%) and 164 (4.4%) secondary malignancies were encountered, respectively (hazards ratio (HR)=1.02, 95% confidence interval (CI): 0.82−1.26, P=0.880 in stratified Cox regression). Figure 1 depicts the cumulative incidence of secondary malignancies for both groups. The HR for breast reconstruction using an implant vs autologous flap for secondary malignancies occurring at least 1 year after diagnosis of breast cancer is outlined in Figure 2. There was no significant association between secondary tumours and breast reconstruction by implants except for lung carcinoma, and this association was substantial when stratified for age (HR=2.51, 95% CI: 1.28–4.96, P=0.005) and when propensity matched (HR=3.22, 95% CI: 1.44–7.20, P=0.002). No significant differences between groups were found for any secondary malignancy including lymphomas (P=0.657 in age-stratified Cox regression). The following lymphoma entities were encountered in the implant group: unspecified lymphoma (N=1), diffuse, large B-cell lymphoma (N=5), follicular lymphoma grade 3 (N=1), cutaneous T-cell lymphoma (N=1), primary cutaneous anaplastic large-cell lymphoma (N=1). The following lymphoma were encountered in the flap group: unspecified lymphoma (N=2), Hodgkin lymphoma with nodular sclerosis (N=1), diffuse, large B-cell lymphoma (N=3), follicular lymphoma grade 2 (N=1), marginal zone B-cell lymphoma (N=4), follicular lymphoma grade 3 (N=1). Combined cardiovascular and pulmonary deaths, including COPD, were significantly more frequent in the implant compared with the flap group (2.3% vs 1.3%, P=0.001). These results were partly confirmed in a sensitivity analysis including 2475 patients with in situ carcinoma of the breast: Overall risk for secondary malignancies after reconstructive breast implants was similar with reconstructive breast implants vs autologous flap (HR=0.96, 95% CI: 0.79−1.16, P=0.665), although there was a numerically increased risk of lung cancer after reconstructive breast implants vs autologous flap using age-stratified Cox regression (HR=1.69, 95% CI:0.97–2.95, P=0.061) or by using propensity score-adjusted Cox regression (HR=1.78, 95% CI:0.96–3.33, P=0.065).
Discussion
We found a significant association between lung cancer and reconstructive breast implants as compared with autologous flap, both by age-stratified Cox regression analysis and propensity score matching. However, we did not find any association between the occurrence of lymphoma and reconstructive breast implants, as previously suggested (Duvic et al, 1995; Keech and Creech, 1997; Center for Devices and Radiological Health, 2011; Jewell et al, 2011; Taylor et al, 2013; Kellogg et al, 2014; Laurent et al, 2016). The average time between first implant placement and the occurrence of breast implant-associated lymphoma was 13.3 years (Locke and Lofts, 2015), moderately longer than the median follow-up time in the present study. To our knowledge, the correlation between lung cancer and breast reconstruction by implants has not been described so far. In the past, numerous epidemiological studies examined the association between cosmetic breast implants and the incidence of cancer (Malone et al, 1992; Bryant and Brasher, 1995; Deapen et al, 1997; Kern et al, 1997; McLaughlin et al, 1998; Brinton et al, 2000; Brinton et al, 2001; Pukkala et al, 2002; Breiting et al, 2004; Friis et al, 2006), and breast silicone implants were declared not to be carcinogenic (Bondurant et al, 1999). Since 2006, however, four retrospective studies have suggested an increased risk of lung cancer among women with cosmetic breast implants, with standardised incidence ratios between 1.6 and 2.2 (McLaughlin et al, 2006; Deapen et al, 2007; Lipworth et al, 2007; Lipworth et al, 2009). The increased lung cancer risk in these women was suggested to be related to the higher prevalence of smoking in women with breast implants, but the present data do not allow to confirm such correlations. The present study has several limitations, including a potential bias due to imbalances between the two study groups despite multivariable analysis and propensity score matching, the presence of unidentified prognostic factors and the fact that the SEER registry does not provide data on cardiovascular risk factors, which may have impacted on the decision to perform autologous flap reconstruction compared with breast implants. In conclusion, the present study shows an increased lung cancer risk in women receiving surgical reconstruction following mastectomy for breast cancer by implants as compared with autologous flaps. At the same time, breast reconstruction by implants is not associated with an increased risk of secondary lymphomas.
Change history
28 June 2016
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Agarwal S, Liu JH, Crisera CA, Buys S, Agarwal JP (2010) Survival in breast cancer patients undergoing immediate breast reconstruction. Breast J 16 (5): 503–509.
Alderman AK, Atisha D, Streu R, Salem B, Gay A, Abrahamse P, Hawley ST (2011) Patterns and correlates of postmastectomy breast reconstruction by US Plastic surgeons: results from a national survey. Plast Reconstr Surg 127 (5): 1796–1803.
Bondurant S, Ernster V, Herdman R (1999) Safety of Silicone Breast Implants. National Academics Press (U.S.): Washington, DC, USA.
Breiting VB, Holmich LR, Brandt B, Fryzek JP, Wolthers MS, Kjoller K, McLaughlin JK, Wiik A, Friis S (2004) Long-term health status of Danish women with silicone breast implants. Plast Reconstr Surg 114 (1): 217–226, discussion 227-8.
Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN (2000) Breast cancer following augmentation mammoplasty (United States). Cancer Causes Control 11 (9): 819–827.
Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN (2001) Cancer risk at sites other than the breast following augmentation mammoplasty. Ann Epidemiol 11 (4): 248–256.
Bryant H, Brasher P (1995) Breast implants and breast cancer—reanalysis of a linkage study. N Engl J Med 332 (23): 1535–1539.
Center for Devices and Radiological Health (2011) Anaplastic Large Cell Lymphoma (ALCL) in Women with Breast Implants: Preliminary FDA Findings and Analysis. FDA.
Chawla AK, Kachnic LA, Taghian AG, Niemierko A, Zapton DT, Powell SN (2002) Radiotherapy and breast reconstruction: complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys 54 (2): 520–526.
Deapen DM, Bernstein L, Brody GS (1997) Are breast implants anticarcinogenic? A 14-year follow-up of the Los Angeles Study. Plast Reconstr Surg 99 (5): 1346–1353.
Deapen DM, Hirsch EM, Brody GS (2007) Cancer risk among Los Angeles women with cosmetic breast implants. Plast Reconstr Surg 119 (7): 1987–1992.
Duvic M, Moore D, Menter A, Vonderheid EC (1995) Cutaneous T-cell lymphoma in association with silicone breast implants. J Am Acad Dermatol 32 (6): 939–942.
Friis S, Holmich LR, McLaughlin JK, Kjoller K, Fryzek JP, Henriksen TF, Olsen JH (2006) Cancer risk among Danish women with cosmetic breast implants. Int J Cancer 118 (4): 998–1003.
Ho D, Imai K, King G, Stuart E (2007) Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 15: 199–236.
Jewell M, Spear SL, Largent J, Oefelein MG, Adams WP Jr (2011) Anaplastic large T-cell lymphoma and breast implants: a review of the literature. Plast Reconstr Surg 128 (3): 651–661.
Keech JA Jr, Creech BJ (1997) Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg 100 (2): 554–555.
Kellogg BC, Hiro ME, Payne WG (2014) Implant-associated anaplastic large cell lymphoma: beyond breast prostheses. Ann Plast Surg 73 (4): 461–464.
Kern KA, Flannery JT, Kuehn PG (1997) Carcinogenic potential of silicone breast implants: a Connecticut statewide study. Plast Reconstr Surg 100 (3): 737–747, discussion 748-9.
Laurent C, Delas A, Gaulard P, Haioun C, Moreau A, Xerri L, Traverse-Glehen A, Rousset T, Quintin-Roue I, Petrella T, Emile JF, Amara N, Rochaix P, Chenard-Neu MP, Tasei AM, Menet E, Chomarat H, Costes V, Andrac-Meyer L, Michiels JF, Chassagne-Clement C, de Leval L, Brousset P, Delsol G, Lamant L (2016) Breast implant-associated anaplastic large cell lymphoma: two distinct clinicopathological variants with different outcomes. Ann Oncol 27 (2): 306–314.
Lin KY, Johns FR, Gibson J, Long M, Drake DB, Moore MM (2001) An outcome study of breast reconstruction: presurgical identification of risk factors for complications. Ann Surg Oncol 8 (7): 586–591.
Lipworth L, Nyren O, Ye W, Fryzek JP, Tarone RE, McLaughlin JK (2007) Excess mortality from suicide and other external causes of death among women with cosmetic breast implants. Ann Plast Surg 59 (2): 119–123, discussion 124-5.
Lipworth L, Tarone RE, Friis S, Ye W, Olsen JH, Nyren O, McLaughlin JK (2009) Cancer among Scandinavian women with cosmetic breast implants: a pooled long-term follow-up study. Int J Cancer 124 (2): 490–493.
Locke MB, Lofts J (2015) Variable presentation of anaplastic large-cell lymphoma in patients with breast implants. ANZ J Surg e-pub ahead of print 1 April 2015 doi:10.1111/ans.13074.
Malone KE, Stanford JL, Daling JR, Voigt LF (1992) Implants and breast cancer. Lancet 339 (8805): 1365.
McLaughlin JK, Lipworth L, Fryzek JP, Ye W, Tarone RE, Nyren O (2006) Long-term cancer risk among Swedish women with cosmetic breast implants: an update of a nationwide study. J Natl Cancer Inst 98 (8): 557–560.
McLaughlin JK, Nyren O, Blot WJ, Yin L, Josefsson S, Fraumeni JF Jr, Adami HO (1998) Cancer risk among women with cosmetic breast implants: a population-based cohort study in Sweden. J Natl Cancer Inst 90 (2): 156–158.
Pukkala E, Boice JD Jr, Hovi SL, Hemminki E, Asko-Seljavaara S, Keskimaki I, McLaughlin JK, Pakkanen M, Teppo L (2002) Incidence of breast and other cancers among Finnish women with cosmetic breast implants, 1970-1999. J Long Term Eff Med Implants 12 (4): 271–279.
Taylor CR, Siddiqi IN, Brody GS (2013) Anaplastic large cell lymphoma occurring in association with breast implants: review of pathologic and immunohistochemical features in 103 cases. Appl Immunohistochem Mol Morphol 21 (1): 13–20.
Tsoi B, Ziolkowski NI, Thoma A, Campbell K, O'Reilly D, Goeree R (2014) Safety of tissue expander/implant versus autologous abdominal tissue breast reconstruction in postmastectomy breast cancer patients: a systematic review and meta-analysis. Plast Reconstr Surg 133 (2): 234–249.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Warschkow, R., Cerny, T., Schmied, B. et al. A population-based analysis of secondary malignancies in breast cancer patients receiving breast reconstruction. Br J Cancer 115, 80–84 (2016). https://doi.org/10.1038/bjc.2016.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2016.108
Keywords
This article is cited by
-
Is Breast Implant Associated—Anaplastic Large Cell Lymphoma linked to textured implants?
Aesthetic Plastic Surgery (2021)