Abstract
Background:
Trend studies investigating the impact of mammographic screening usually display age-specific mortality and incidence rates over time, resulting in an underestimate of the benefit of screening, that is, mortality reduction, and an overestimate of its major harmful effect, that is, overdiagnosis. This study proposes a more appropriate way of analysing trends.
Methods:
Breast cancer mortality (1950–2009) and incidence data (1975–2009) were obtained from Statistics Netherlands, ‘Stg. Medische registratie’ and the National Cancer Registry in the Netherlands for women aged 25–85 years. Data were visualised in age–birth cohort and age–period figures.
Results:
Birth cohorts invited to participate in the mammographic screening programme showed a deflection in the breast cancer mortality rates within the first 5 years after invitation. Thereafter, the mortality rate increased, although less rapidly than in uninvited birth cohorts. Furthermore, invited birth cohorts showed a sharp increase in invasive breast cancer incidence rate during the first 5 years of invitation, followed by a moderate increase during the following screening years and a decline after passing the upper age limit.
Conclusion:
When applying a trend study to estimate the impact of mammographic screening, we recommend using a birth cohort approach.
Similar content being viewed by others
Main
In a recent poll, a quarter of the European clinicians voted against recommending routine mammographic screening, as they believed that the benefits of screening in terms of mortality reduction did not outweigh the harms resulting from screening (Colbert and Adler, 2013). This is remarkable, considering that most randomised controlled trials and observational studies have shown large mortality reductions since the introduction of mammographic screening (Broeders et al, 2012). Trend studies are often executed to evaluate the impact of mammographic screening on breast cancer mortality. To this end, they usually display age-specific rates of breast cancer death for calendar periods (Ascunce et al, 2007; Haukka et al, 2011; Mukhtar et al, 2013). The observed mortality decreases in these studies ranged between 1% and 9% annually (Moss et al, 2012). These percentages are likely to be diluted, because the effect of screening on breast cancer mortality is small shortly after women enter the mammographic screening programme and endures after women leave the screening programme (Moss et al, 2012). In other words, the effect of mammographic screening on mortality is not restricted to the screened age range but is dependent on whether or not women are screened.
A similar condition applies to the effect of mammographic screening on the breast cancer incidence. Mammographic screening has immediate effects, such as the increased incidence directly after introduction, and delayed effects that can only be observed in women who have left the screening programme, such as the compensatory drop (Boer et al, 1994). In order to be able to make a reliable estimate of the main harmful effect of screening, that is, overdiagnosis, both immediate and delayed effects of mammographic screening on breast cancer incidence should be taken into account.
This study aims to provide more insights into both the immediate and delayed effects of mammographic screening by visualising trends in breast cancer mortality and incidence in ‘birth cohorts not invited’ and ‘birth cohorts invited’ to participate in the Dutch mammographic screening programme.
Materials and methods
The Dutch screening programme
In 1989, the implementation of the population-based biennial mammography screening programme in the Netherlands started, inviting all women aged 50–69 years. The geographic coverage of the screening programme increased from 11% in 1990, to 26% in 1991, 48% in 1992, 69% in 1993, 77% in 1994, 88% in 1995 and to its full capacity in 1996 (Otten et al, 2008). The attendance during this implementation period increased from 72.5% in 1990 to 80.1% in 1997; thereafter, it remained stable at around 80% (National Evaluation Team for Breast Cancer Screening, 2012). In 1997, the screening programme was extended to include women aged 70–74 years. The geographic coverage of the screening programme of the age group of 70–74 years increased from 26% in 1998, to 86% in 1999, 91% in 2000 and to its full coverage in 2001 (Otten et al, 2008). Furthermore, the percentage of definite non-participants, that is, those who do not wish to receive further invitations to participate in the screening programme, has always been low (2.0–7.5%; National Evaluation Team for Breast Cancer Screening, 2009). From mid-2004 to 2010, there was a transition from screen-film mammography to digital mammography (National Evaluation Team for Breast Cancer Screening, 2009).
Breast cancer mortality and incidence data
Data on the female population and on the number of breast cancer deaths were obtained per calendar year (1950–2010) in 5-year age groups from Statistics Netherlands (Centraal Bureau voor de Statistiek, 2012). Data on invasive breast cancer incidence were obtained from the Stg. Medische Registratie (1975–1988) and the website of the National Cancer Registry in the Netherlands (1989–2010) (Integraal kankercentrum Nederland, 2012). We limited our analyses to invasive breast cancer, because the incidence of carcinoma in situ (CIS) was not registered before 1989. Carcinoma in situ represented 4.4% of all newly diagnosed breast cancers in 1989 and 13.1% in 2010. The exclusion of CIS resulted in this study’s results in an under-representation of the actual number of new breast cancer cases that are normally presented for the Netherlands.
Crude breast cancer mortality and invasive breast cancer incidence rates were calculated per 100 000 women years using the female mid-population as denominator. Birth cohorts were computed by subtracting the age at breast cancer death or diagnosis from the calendar period. As age was obtained in 5-year groups and calendar period was aggregated in 5-year groups, the birth cohorts cover 10 overlapping years and are indicated by the 5 middle years. Figures were produced with Microsoft Excel (2007) using 5-year averages in the age–birth cohort figures and 5-year moving averages in the age–period figure to reduce large annual fluctuations.
Results
Figure 1 displays the breast cancer mortality rates per birth cohort for cohorts of women that never participated in the national mammographic screening programme (Figure 1A) and for cohorts of those women invited to participate in the screening programme (Figure 1B). The birth cohorts not exposed to the national mammographic screening programme all show an increasing breast cancer mortality rate with age, which is similar for all birth cohorts. Younger birth cohorts also show a slightly higher breast cancer mortality rate than older birth cohorts. The birth cohorts not displayed in Figure 1A (to facilitate interpretation of trends) showed the same pattern.
Breast cancer mortality rates per 100 000 women years for birth cohorts ( A ) uninvited and ( B ) invited to participate in the national mammography screening programme. Women born between 1923 and 1927 were 65–69 years old when invited to mammography screening for the first time. Women born between 1928 and 1932 were first invited at the age of 60–64 years, women born between 1933 and 1937 were first invited at the age of 55–59 years and women born after 1938 were invited for the first time when they reached the age of 50 years.
In comparison, the invited birth cohorts (Figure 1B) show a deflection in the breast cancer mortality rate that starts within the first 5 years after receiving an invitation to participate in the mammographic screening programme. After this initial deflection, the mortality rate increases again, although at a much lower rate in comparison with women of the same age in the uninvited birth cohorts. These effects are more prominent in those birth cohorts invited more often to participate in the mammographic screening programme.
Figure 1B also shows that the breast cancer mortality rate is lower in younger birth cohorts in both the age range where women are invited to participate in the national screening programme (older than 50 years) and the age range before women enter the national screening programme (younger than 50 years).
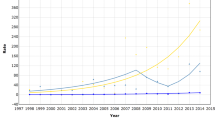
Figure 2 presents age-specific breast cancer mortality rates over time. Overall, the breast cancer mortality increased from 1950 to 1990, which corresponds to the increasing mortality in younger uninvited birth cohorts (Figure 1A). After the implementation of mammographic screening in 1990 for women aged 50–69 years, breast cancer mortality starts to decline in women younger than 70 years, although very little at the start. From 1999 onwards, the breast cancer mortality also starts to decline in women aged 70–84 years. When taking the opportunity for screening into account, Figure 2 indicates that the mortality decline increases with increasing age. To facilitate comparison of Figure 2 with Figure 1, three birth cohorts, indicated by their middle year, were added to Figure 2. The lines of the birth cohorts are fairly parallel before the implementation of mammographic screening and start to deviate from each other after the implementation of mammographic screening.
Age-specific breast cancer mortality rates per 100 000 women years over time. Women born between 1908 and 1912 were never invited for mammographic screening. Women born between 1923 and 1927 were first invited at the age of 65–69 years and women born between 1938 and 1942 were first invited at the age of 50–54 years.
Figure 3 shows the invasive breast cancer incidence rates per birth cohort for cohorts of women that never participated in the mammographic screening programme (Figure 3A) and for cohorts of women invited to participate in the screening programme (Figure 3B and C). The uninvited birth cohorts show an increasing invasive breast cancer incidence rate with increasing age, where the younger birth cohorts have a higher incidence than the older birth cohorts. The invited birth cohorts show a large peak in the invasive breast cancer incidence at the age of first invitation, which is followed by moderate increases in the birth cohorts of 1933–1937 and younger. The birth cohort of 1923–1927 shows a drop in the invasive breast cancer rate in the age group of 70–79 years, when women in this birth cohort left the screening programme, and, thereafter, an increase in the invasive breast cancer rate. The 1928–1932 birth cohort shows a drop at the age 75–79 years that is more prominent than the drop of the 1923–1927 birth cohort.
Invasive breast cancer incidence rates per 100 000 women years for birth cohorts ( A ) uninvited, ( B ) invited at age 55 years or older and ( C ) invited since age 50 years to participate in the national mammographic screening programme. Women born between 1923 and 1927 were 65–69 years old when invited to mammography screening for the first time, and are thereby the eldest birth cohort invited to screening. Women born between 1928 and 1932 were first invited at the age of 60–64 years, women born between 1933 and 1937 were first invited at the age of 55–59 years and women born after 1938 were invited for the first time when they reached the age of 50 years.
Figure 3A–C all show that the invasive breast cancer incidence rate is higher in younger birth cohorts, irrespective of their being invited to participate in the mammographic screening programme.
Discussion
Birth cohorts invited for mammographic screening had lower breast cancer mortality rates than uninvited birth cohorts; the breast cancer mortality rates in the invited and uninvited birth cohorts started to diverge shortly after the introduction of the mammographic screening programme and continued to diverge after the upper age limit of eligibility was reached. In addition, birth cohorts invited for mammographic screening showed a sharp increase in the invasive breast cancer incidence at age of first invitation and a drop in the invasive breast cancer incidence after the upper age limit of eligibility was reached.
Trend studies evaluating the impact of mammographic screening have found a decreasing (age-specific) breast cancer mortality rate over time (Moss et al, 2012). The results of our study corresponds these findings, showing a decreasing age-specific breast cancer mortality rate over time after the introduction of mammographic screening and a decreasing breast cancer mortality rate in birth cohort invited to participate in the mammographic screening programme.
Trend studies are often used to quantify the impact of mammographic screening on breast cancer mortality. So far, most studies have compared a screening period with a non-screening period, or screened age groups with pre- and post-screening age groups (Moss et al, 2012). Some trend studies have also tried to take the delayed effects of mammographic screening into account by excluding women in the first 5 years of starting screening (Otten et al, 2008; Moss et al, 2012). However, this study suggests that trends of birth cohorts are preferable to trends over time when studying the impact of mammographic screening on breast cancer mortality. First, trend studies comparing a screening period with a non-screening period are likely to dilute the impact of mammographic screening, as this study shows that the breast cancer mortality increases slightly with birth cohort and over time. In addition, changes in other factors, such as therapy, make comparisons over time difficult. Second, trend studies comparing screened age groups with pre- and post-screening age groups are also likely to dilute the impact of mammographic screening, as this study shows that the breast cancer mortality rates in post-screening age groups are lower in invited birth cohorts than in uninvited birth cohorts. Lastly, exclusion of women in the first 5 years from the start of screening can partly limit dilution of the impact of mammographic screening, although it cannot take into account that the impact of mammographic screening on breast cancer mortality is gradual. Therefore, we recommend studying the impact of mammographic screening in the same group of women, that is, by comparing invited birth cohorts with uninvited birth cohorts.
Even though birth cohorts can account for the gradual impact of mammographic screening on breast cancer mortality trends, it remains difficult to interpret mortality trends. This study shows that the breast cancer mortality rate decreases with successive birth cohorts after the implementation of mammographic screening, regardless of being invited to participate in the mammographic screening programme. In the non-invited birth cohorts, the decreasing breast cancer mortality can be explained by, among others, advances in breast cancer therapy and increased awareness, whereas it is likely to be caused by a combination of screening, therapy and other factors in the invited birth cohorts. Some authors have analysed and estimated the individual effects of mammography screening and therapy on the mortality decrease in invited age groups, which has resulted in estimates varying from 20% to 72% for adjuvant systemic therapy and from 28% to 80% for mammography screening (Blanks et al, 2000; Vervoort et al, 2004; Berry et al, 2005; De Gelder, 2012).
The birth cohorts invited for mammographic screening showed a sharp short-term increase in the invasive breast cancer incidence rate followed by more moderate increases. This is a well-known phenomenon, which has been demonstrated in studies displaying age–cohort graphics (Brown et al, 2009; Martinez-Alonso et al, 2010; Junod et al, 2011) or age–period graphics (Svendsen et al, 2006; Otten et al, 2008; Pollán et al, 2010).
When invited birth cohorts exceed the upper age limit of the screening programme, the invasive breast cancer incidence rate drops to a much lower level than would be expected at that age in a non-screening situation. This is in line with the prediction by Boer et al (1994) and the results of other studies (De Gelder et al, 2011). For a reliable calculation of the percentage of overdiagnosis, this drop in incidence after leaving the screening programme should be taken into account in addition to the extra incidence during the screening programme. Preferably, overdiagnosis should be calculated from the extra incidence and drop in one birth cohort of women, as our results suggest that the magnitude of the drop depends on how long the women were invited to participate in mammographic screening. Until now, only a few studies have used a cohort approach to study overdiagnosis, leading to estimates ranging from 1% to 5% (Puliti et al, 2011; Njor et al, 2013).
Younger birth cohorts show a higher invasive breast cancer incidence rate irrespective of being invited to participate into the mammographic screening programme. Other studies have also found an increasing breast cancer incidence rate with younger birth cohorts, even when adjusted for the introduction of mammographic screening (Brown et al, 2009; Martinez-Alonso et al, 2010; Viel et al, 2011). This increase in incidence might be explained by increasing risk factors (Brown et al, 2009), such as age at first birth, and/or changes in diagnostic practice (Viel et al, 2011). Although it is difficult to disentangle the combined effects, our study suggests that other forms of early detection have a role in the increasing breast cancer incidence, because this coincides with a decreasing breast cancer mortality rate. Factors contributing to early detection of breast cancer in the Netherlands are increased awareness, increased use of opportunistic mammography and/or counselling and detection of high-risk families since the late 1990s (National Evaluation Team for Breast Cancer Screening, 2009).
In this study, we chose to visualise trends in the breast cancer mortality and invasive breast cancer incidence in birth cohorts of women invited and not invited to participate in the mammographic screening programme. A major advantage of age–cohort graphics in comparison with the more common age-specific rates in time periods is that effects of changes, such as the introduction of mammographic screening, can be followed in one group of women over time, that is, it displays both immediate and delayed effects. Furthermore, this approach is most suitable for visualising the effects of mammographic screening on breast cancer incidence for other factors influencing breast cancer incidence, for example, risk factors, do not depend on the same age and cohort combination as mammographic screening.
We chose not to estimate the effect of age, period and birth cohort, as there is no unique parameterisation of these three parameters, that is, a ‘non-identifiability’ problem, without making assumptions (Clayton and Schifflers, 1987a, 1987b; Holford, 1991; Tarone and Chu, 1992). In addition, we believed that the message would be clearer by visualising breast cancer mortality and incidence trends in birth cohorts rather than by quantifying the effects.
To conclude, mammographic screening has both immediate and delayed effects on the breast cancer mortality and incidence. A birth cohort approach prevents underestimation of the mortality reduction by taking into account the effects of screening above the upper age limit for screening and by not including constant additions of women newly entering the screening programme. In addition, it prevents overestimation of overdiagnosis, because this can be calculated from the extra incidence and its associated compensatory drop. Therefore, we recommend using a birth cohort approach when designing trend studies to estimate the impact of mammographic screening.
Change history
29 October 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Ascunce EN, Moreno-Iribas C, Urtiaga AB, Ardanaz E, Sanz ME, Castilla J, Egüés N (2007) Changes in breast cancer mortality in Navarre (Spain) after introduction of a screening programme. J Med Screen 14 (1): 14–20.
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JDF, Feuer EJ (2005) Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 353 (17): 1784–1792.
Blanks RG, Moss SM, McGahan CE, Quinn MJ, Babb PJ (2000) Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990-8: comparison of observed with predicted mortality. Br Med J 321 (7262): 665.
Boer R, Warmerdam P, De Koning H, Van Oortmarssen G (1994) Extra incidence caused by mammographic screening. Lancet 343 (8903): 979.
Broeders M, Moss S, Nystrom L, Njor S, Jonsson H, Poop E, Massat N, Duffy S, Lynge E, Paci E Euroscreen Working Group (2012) The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J Med Screen 19: 14–25.
Brown SBF, Morrison DS, Cooke TG (2009) Increasing incidence of breast cancer: distinguishing between the effects of birth cohort and a national breast screening programme. Breast Cancer Res Treat 116 (3): 603–607.
Centraal Bureau voor de Statistiek (2012) Available at statline.cbs.nl (accessed: 18 June 2012).
Clayton D, Schifflers E (1987a) Models for temporal variation in cancer rates. I: Age-period and age-cohort models. Stat Med 6 (4): 449–467.
Clayton D, Schifflers E (1987b) Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat Med 6 (4): 469–481.
Colbert JA, Adler JN (2013) Clinical decisions. Mammography screening—polling results. N Engl J Med 368 (9): e12.
De Gelder R (2012) Predicting the Benefits and Harms of Breast Cancer Screening: Current Debates and Future Directions (Doctoral). Erasmus University: Rotterdam.
De Gelder R, Heijnsdijk EAM, Van Ravesteyn NT, Fracheboud J, Draisma G, De Koning HJ (2011) Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol Rev 33 (1): 111–121.
Haukka J, Byrnes G, Boniol M, Autier P (2011) Trends in breast cancer mortality in Sweden before and after implementation of mammography screening. PLoS One 6 (9): e22422.
Holford TR (1991) Understanding the effects of age, period, and cohort on incidence and mortality-rates. Annu Rev Public Health 12: 425–457.
Integraal kankercentrum Nederland (2012) Available at www.cijfersoverkanker.nl (accessed: 18 June 2012).
Junod B, Zahl PH, Kaplan RM, Olsen J, Greenland S (2011) An investigation of the apparent breast cancer epidemic in France: screening and incidence trends in birth cohorts. BMC Cancer 11 (401)).
Martinez-Alonso M, Vilaprinyo E, Marcos-Gragera R, Rue M (2010) Breast cancer incidence and overdiagnosis in Catalonia (Spain). Breast Cancer Res 12 (4): R58.
Moss SM, Nystrom L, Jonsson H, Paci E, Lynge E, Njor S, Broeders M Euroscreen Working Group (2012) The impact of mammographic screening on breast cancer mortality in Europe: a review of trend studies. J Med Screen 19: 26–32.
Mukhtar TK, Yeates DR, Goldacre MJ (2013) Breast cancer mortality trends in England and the assessment of the effectiveness of mammography screening: population-based study. J R Soc Med 106 (6): 234–242.
National Evaluation Team for Breast Cancer Screening (2009) National Evaluation of Breast Cancer Screening in The Netherlands. 1990–2007 (XII). Twelfth Evaluation Report. Erasmus MC: Rotterdam.
National Evaluation Team for Breast Cancer Screening (2012) Main Results 2010 Breast Cancer Screening Programme in The Netherlands. Erasmus MC: Rotterdam.
Njor SH, Olsen AH, Blichert-Toft M, Schwartz W, Vejborg I, Lynge E (2013) Overdiagnosis in screening mammography in Denmark: population based cohort study. BMJ 346: f1064.
Otten JDM, Broeders MJM, Fracheboud J, Otto SJ, De Koning HJ, Verbeek ALM (2008) Impressive time-related influence of the Dutch screening programme on breast cancer incidence and mortality, 1975-2006. Int J Cancer 123 (8): 1929–1934.
Pollán M, Michelena MJ, Ardanaz E, Izquierdo A, Sánchez-Pérez MJ, Torrella A, Vicente Argüelles-Suárez M, Borràs J, Franch P, Jiménez R, Navarro C, Rabanaque MJ, Ramírez C, Rojas MD, Sáinz-Miranda M (2010) Breast cancer incidence in Spain before, during and after the implementation of screening programmes. Ann Oncol 21 (Suppl 3): iii97–iii102.
Puliti D, Miccinesi G, Paci E (2011) Overdiagnosis in breast cancer: design and methods of estimation in observational studies. Prevent Med 53 (3): 131–133.
Svendsen AL, Olsen AH, Von Euler-Chelpin M, Lynge E (2006) Breast cancer incidence after the introduction of mammography screening: What should be expected? Cancer 106 (9): 1883–1890.
Tarone RE, Chu KC (1992) Implications of birth cohort patterns in interpreting trends in breast cancer rates. J Natl Cancer Inst 84 (18): 1402–1410.
Vervoort MM, Draisma G, Fracheboud J, van de Poll-Franse LV, de Koning HJ (2004) Trends in the usage of adjuvant systemic therapy for breast cancer in the Netherlands and its effect on mortality. Br J Cancer 91 (2): 242–247.
Viel JF, Rymzhanova R, Fournier E, Danzon A (2011) Trends in invasive breast cancer incidence among French women not exposed to organized mammography screening: An age-period-cohort analysis. Cancer Epidemiol 35 (6): 521–525.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ripping, T., Verbeek, A., van der Waal, D. et al. Immediate and delayed effects of mammographic screening on breast cancer mortality and incidence in birth cohorts. Br J Cancer 109, 2467–2471 (2013). https://doi.org/10.1038/bjc.2013.627
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.627
Keywords
This article is cited by
-
Zeitliche Entwicklung der Programmsensitivität des deutschen Mammographie-Screening-Programms in Nordrhein-Westfalen und Niedersachsen
Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz (2018)