Abstract
Background:
Change in breast density may predict outcome of women receiving adjuvant hormone therapy for breast cancer. We performed a prospective clinical trial to evaluate the impact of inherited variants in genes involved in oestrogen metabolism and signalling on change in mammographic percent density (MPD) with aromatase inhibitor (AI) therapy.
Methods:
Postmenopausal women with breast cancer who were initiating adjuvant AI therapy were enrolled onto a multicentre, randomised clinical trial of exemestane vs letrozole, designed to identify associations between AI-induced change in MPD and single-nucleotide polymorphisms in candidate genes. Subjects underwent unilateral craniocaudal mammography before and following 24 months of treatment.
Results:
Of the 503 enrolled subjects, 259 had both paired mammograms at baseline and following 24 months of treatment and evaluable DNA. We observed a statistically significant decrease in mean MPD from 17.1 to 15.1% (P<0.001), more pronounced in women with baseline MPD ⩾20%. No AI-specific difference in change in MPD was identified. No significant associations between change in MPD and inherited genetic variants were observed.
Conclusion:
Subjects with higher baseline MPD had a greater average decrease in MPD with AI therapy. There does not appear to be a substantial effect of inherited variants in biologically selected candidate genes.
Similar content being viewed by others
Main
There is considerable interpatient variation in the mammographic appearance of the breast, in part because of differences in the characteristics of different components of breast tissue (Pinsky and Helvie, 2010). Fibroglandular tissue absorbs more of the X-ray beam and therefore appears light, whereas fat absorbs less of the X-ray beam and is darker. In clinical practice, mammographic percent density (MPD) has been estimated by radiologists using visual assessment to categorise percent breast density into quartiles (American College of Radiology, 2003). Methods have also been developed to quantitate MPD, including manual, computer-aided, and fully computerised methodologies (Byng et al, 1994; Boyd et al, 1995; Zhou et al, 2001; Wei et al, 2004; Martin et al, 2006).
High breast density as assessed by mammography is one of the strongest risk factors for breast cancer (Boyd et al, 2005). Factors definitely associated with breast density include age, menopausal status, body mass index (BMI), and exogenous hormone use. In general, MPD declines with increasing age, during the menopausal transition, and with increasing body weight (Boyd et al, 1998; Vachon et al, 2000; Martin and Boyd, 2008). Exposure to steroidal sex hormones may also play a role in an individual’s MPD. Numerous reports have demonstrated increased MPD with exposure to hormone replacement therapy (Rutter et al, 2001; Boyd et al, 2006). However, reports from studies evaluating associations between endogenous circulating hormone concentrations and MPD have yielded mixed results (Aiello et al, 2005; Greendale et al, 2005; Tamimi et al, 2005; Warren et al, 2006; Martin and Boyd, 2008).
Treatment with the selective oestrogen receptor modulator tamoxifen has been shown to decrease MPD, especially in women aged ⩽45 years (Cuzick et al, 2004). Benefit from tamoxifen has been shown to be greater in women with a greater reduction in MPD (Cuzick et al, 2011; Kim et al, 2012). It is unknown whether a similar association between decrease in MPD and decreased risk of breast cancer recurrence is present in postmenopausal women treated with aromatase inhibitor (AI) therapy.
Aromatase is a key enzyme required for the final step in the conversion of androgens to oestrogens. Aromatase inhibitors, which inhibit the production of oestrogen, have been shown to decrease the risk of new primary breast cancer and breast cancer recurrence in postmenopausal women (Burstein et al, 2010; Dowsett et al, 2010; Goss et al, 2011). However, the impact of AI therapy on MPD is uncertain. Two small studies evaluating the impact of AI therapy on MPD found no change over 24 months (Cigler et al, 2010, 2011), and another detected a nonstatistically significant decrease in MPD over 12 months (Prowell et al, 2011). A case–control study demonstrated a >5% reduction in MPD in 14% of 387 women treated with AI therapy for an average of 10 months, which was not statistically different from matched controls (Vachon et al, 2013).
Population-based studies have demonstrated that genetic effects can affect MPD (Boyd et al, 2002; Douglas et al, 2008; Ursin et al, 2009; Greenwood et al, 2011; Varghese et al, 2012). In twins, heritable factors account for about two-thirds of the variation in MPD (Boyd et al, 2002). However, few specific inherited variants have been identified to be associated with MPD (Haiman et al, 2003; Warren et al, 2006; Olson et al, 2007; Li et al, 2010; Lindstrom et al, 2011; Ellingjord-Dale et al, 2012). Therefore, although there appears to be a heritable component to MPD, the contributing genetic variants have not been fully elucidated.
We conducted a prospective randomised trial to test the effects on MPD of two AIs (letrozole (Femara, Novartis, Basel, Switzerland) vs exemestane (Aromasin, Pfizer, New York, NY, USA)) in postmenopausal women with early-stage breast cancer who were initiating adjuvant AI therapy. Mammographic percent density was assessed using a validated computer-assisted method (Martin et al, 2006; Douglas et al, 2008) and included multiple prospective quality measures to minimise bias. The primary objectives of the study were to determine the changes in MPD following 24 months of AI therapy and to determine whether the change in MPD is correlated with genetic variants in CYP19A1, the gene that encodes aromatase.
Materials and methods
Eligible patients were recruited from August 2005 through July 2009 to the prospective Exemestane and Letrozole Pharmacogenomics (ELPh) trial (clinicaltrials.gov no. NCT00228956). This trial was conducted by the Consortium on Breast Cancer Pharmacogenomics (COBRA), which includes the Indiana University Bren and Melvin Simon Cancer Center (IU), the University of Michigan Comprehensive Cancer Center (UM), and the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University (JH). Detailed inclusion and exclusion criteria have previously been described (Henry et al, 2008). In brief, postmenopausal women with stage 0–III hormone receptor-positive breast cancer were eligible. Patients were excluded if they had bilateral mastectomy or prior radiation to the contralateral (unaffected) breast. All indicated surgery, chemotherapy, and radiation therapy for breast cancer were completed before enrollment. Prior tamoxifen was permitted. The protocol was approved by the Institutional Review Boards of all participating study sites (JH, IU and UM), and all enrolled patients provided written informed consent. The clinical trial was reviewed by an independent Data and Safety Monitoring Committee on a bi-annual basis.
Study design
Following enrollment, women were randomly assigned to treatment with exemestane (25 mg) or letrozole (2.5 mg) daily for 2 years. Randomisation was stratified based on prior tamoxifen (yes/no), prior chemotherapy (yes/no), and bisphosphonate therapy (yes/no). Patients underwent a single craniocaudal view mammogram of the contralateral breast at the baseline and 24 month visits. Whole blood was collected at the baseline visit for DNA extraction and genotyping. Serum samples were obtained at baseline and after 3 months of AI therapy to determine oestrogen concentrations.
Mammographic assessment
Two study sites acquired mammographic images using General Electric machines (Fairfield, CT, USA) (JH and UM) and the other site used a Hologic machine (Bedford, MA, USA) (IU). During the conduct of the study, the study sites transitioned from film mammography to digital mammography following the results of the Digital Mammographic Imaging Screening Trial (Pisano et al, 2005). However, with a few exceptions, each patient was evaluated with the same technology (film or digital mammography) at both time points. Film mammograms were digitised with a laser scanner designed for mammogram digitisation. If patients had digital mammograms, raw data were obtained and sent to the radiologist for processing and analysis whenever possible. Mammograms were excluded from analysis if only processed digital data were available or if presence of breast implants prevented accurate assessment of MPD. Although the mammograms were performed for research purposes, all mammographic images were reviewed by a radiologist (MAH and RP) within 45 days of the procedure to confirm that there were no clinically relevant findings. If such findings were detected, they were provided to the caregiver for appropriate evaluation.
In order to avoid interobserver variability, a single Mammographic Quality Standards Act-approved breast radiologist (MAH) with more than 25 years of experience in mammography performed all density assessments within a short period of time at the conclusion of the clinical trial, blinded to patient number, time point, and AI medication. This reader, who had prior experience using the assessment method, underwent training on a learning set of images immediately before analysing the cases in this study. As previously reported, the within-individual r for assessment of percent density using this technique was 0.96, and the mean absolute difference in intrareader variability was 3.5% with a root mean squared error of 4.5% (Douglas et al, 2008).
Mammographic percent density was assessed for each mammogram using two different methodologies. The radiologist first evaluated all the mammograms using the Breast Imaging Reporting and Data System (BIRADS) density classification, in which mammograms are grouped using visual estimation of MPD into four quartiles ranging from category 1 (<25% fibroglandular) to category 4 (>75% fibroglandular). After completion of all of the BIRADS assessments, the radiologist then interactively used the computer program Mammographic Density ESTimator (MDEST) to quantitatively estimate the MPD as the Reference Standard. The MDEST enhances the image, detects the breast boundary, and analyses the grey-level histogram as previously reported (Zhou et al, 2001). The dense area is then segmented using adaptive thresholding techniques and its percentage relative to the breast area is calculated. The radiologist visually compared the original and segmented mammograms on the display workstation, and could interactively adjust the threshold for segmentation and/or breast border chosen by the computer if necessary. True dense area was determined for each mammogram by multiplying percent density by the total breast area. The value is given in mm2, based on a pixel size of 0.8 mm by 0.8 mm. The Reference Standard is the method that was prospectively identified in the clinical protocol as the method that would be used for the analysis of the primary objective.
Sample processing and genotyping
Serum was obtained from each subject at baseline and after 3 months of AI therapy, when drug levels would have reached steady state. Samples were analysed for oestrogen and its metabolites, including oestrone sulphate (E1S), using gas chromatography tandem mass spectroscopy (PharmaNet, Richboro, PA, USA). In brief, analytes and deuterated internal standards were extracted from 0.4 ml of human plasma using BondElut Certify solid-phase cartridges (Agilent Technologies, Santa Clara, CA, USA). The E1S was eluted from the cartridges with ethyl acetate and then underwent three separate derivatisations: (1) reaction with pentafluorobenzoyl chloride, (2) reaction with O-(2,3,4,5,6-pentaflurorobenzyl)-hydroxylamine hydrochloride, and (3) reaction with MSTFA. The derivatised E1S was then separated by gas chromatography and detected by tandem mass spectrometry using negative ion chemical ionisation. The lower limit of quantification (LOQ) was 3.13 pg ml−1, and the upper LOQ was 800 pg ml−1.
Whole blood was collected from each participant at the baseline study visit. The DNA was extracted from whole blood using Qiafilter Blood DNA Maxi kit (Qiagen, Inc., Valencia, CA, USA). At the time of protocol development, candidate single-nucleotide polymorphisms (SNPs) in 24 genes were identified through a priori review of the literature based on their potential functional significance in a variety of AI-associated effects, including change in breast density, change in bone density, and development of treatment-emergent symptoms including arthralgias and hot flashes. Before conducting the analysis of associations between genetic variants and change in breast density reported in this paper, the decision was made by the Investigators to limit the analysis to the 13 genes that we identified as playing a biologic role in the development or maintenance of breast density. Therefore, only SNPs in genes involved in oestrogen metabolism (COMT, CYP19A1), oestrogen receptor (ER) signalling (ESR1, ESR2, PGR), AI drug metabolism (CYP2A6, CYP3A5), and coregulation of ER (EP300, NCOA1, NCOA2, NCOA3, NCOR1, NCOR2) were included; SNPs in EZH2, NRIP, PELP1, and neurotransmitter and neuropeptide signalling (HTR1A, HTR2A, SCL6A4, HCRT, HCRTR1, HCRTR2) were excluded. In total, 135 candidate variants in 13 individual genes were identified and genotyped. One variant (rs9340941) that did not meet Hardy–Weinberg equilibrium, one (rs1256066) for which genotype could not be determined in >80% of subjects, and 6 (rs9322348, rs9340872, rs9341069, rs9341074, rs9341081, rs9341084) with a minor allele frequency (MAF) of 0 were excluded from the analysis. A total of 127 variants in 13 genes were included in the final analysis. The rs numbers, MAF, and genotype frequencies for each analysed SNP are listed in Supplementary Tables S1 and S2.
Genotyping for all SNPs, except for the CYP3A5 and CYP2A6 SNPs, was performed using the BioTroveOpenArray platform (Applied Biosystems, Inc., Foster City, CA, USA). The CYP3A5 *3 was genotyped using the Taqman assay (Life Technologies, Carlsbad, CA, USA) (C__26201809_30). Genotyping for CYP2A6 was performed as previously described (Desta et al, 2011). Genotype quality control was performed before analysis of the genetic associations. For quality control purposes, ∼10% of the samples were randomly selected and genotyped in duplicate using the same assay, and the overall concordance rate was 97%.
Statistical analysis
The primary end point of the ELPh trial was the correlation between absolute change in MPD with 2 years of AI therapy (either exemestane or letrozole) and inherited genetic variants in the aromatase gene, encoded by CYP19. Our initial power and sample size calculation was based on a 20% dropout rate among 500 recruited patients. We estimated the power to be 81% in order to detect an absolute 1.0% change in MPD with 2 years of AI therapy, with a 7% s.d. The power to detect a similar size of CYP19 dominance effect on both drugs’ effect on MPD is 42%, given a MAF of 0.20. The type I error in both tests was controlled at 5% each. After the study was concluded, we had data from only 259 patients to test the drug effect and genetic effect hypotheses, which reduced the power to 63% and 20%, respectively.
Initially, mean MPD and true dense area was determined for all subjects at baseline, as well as for subjects with available paired baseline and 24-month mammograms. Percentage change in MPD was defined as (24-month MPD minus baseline MPD) divided by baseline MPD. Percentage change in true dense area was defined as (24-month true dense area minus baseline true dense area) divided by baseline true dense area. Mean percentage change for either MPD or true dense area was calculated by averaging the individual percentage change values for each subject. The t-tests were used to compare MPD and true dense area between baseline and 24 months in all subjects. Univariate and multivariate analyses were performed to study effects of covariates on baseline MPD or true dense area and change in MPD or true dense area. The E1S values that were below the lower LOQ were changed to the lower LOQ (3.13 pg ml−1), and those values that were above the upper LOQ were changed to the upper LOQ (800 pg ml−1).
Hardy–Weinberg disequilibrium tests were conducted for each SNP, with a P-value threshold of 0.001 to account for the multiple testing. The associations between the candidate SNPs and either baseline or absolute change in MPD with 2 years of AI therapy were analysed either without or with justifying for BMI and/or prior chemotherapy. Three genetic models, specifically dominant, recessive, and additive, were used to test for the associations between SNPs and MPD or true dense area. For analyses performed using the recessive model, SNPs with ⩽5 subjects homozygous for the variant allele were excluded. For patients with 2-year follow-up data, absolute change in breast density was used as the dependent variable. We used PLINK software (http://pngu.mgh.harvard.edu/purcell/plink) to perform genotyping data analysis. Default parameters were used for PLINK software. In order to account for multiple genetic comparisons with 127 SNPs, statistical significance was defined as a P-value of <0.00039 based on Bonferroni correction.
Results
Patient characteristics
A total of 503 subjects enrolled on the ELPh trial (Figure 1). From the baseline analyses, 94 subjects were excluded for the following reasons: (1) technical error (n=1), (2) breast implants prevented accurate assessment of breast density (n=6), (3) no mammography at baseline (n=4), and (4) no raw data available (either film mammograms were digitised or only processed digital mammogram data were available; n=83). Therefore, a total of 409 subjects had evaluable baseline mammograms with raw digital data available for assessment. Genotype data were available for 385 of the 409 subjects (94.1%) with baseline mammograms. The other 24 subjects did not have DNA available for a variety of reasons, including technical errors, inability to obtain blood at baseline, or insufficient quantity of DNA.
Of the 503 subjects enrolled on the ELPh trial, 299 (59.4%) completed at least 18 months of AI therapy on study and were potentially evaluable for the primary end point. The remaining 204 (40.6%) subjects discontinued study participation before 18 months, principally because of toxicities of the AI to which they were assigned, as previously described (Henry et al, 2012). Of the 299 subjects, 26 were excluded for the following reasons: (1) no mammography at 24 months (n=15), (2) breast implants that confounded interpretation of breast density (n=6), (3) technical error (n=1), and (4) film mammogram at baseline and digital mammogram at 24 months (n=4). Therefore, 273 subjects who remained on the assigned treatment had evaluable mammograms at both baseline and 24 months, and genotype data were available for 259 (94.9%) of these subjects (51.5% of initially enrolled subjects).
Clinical characteristics of the evaluable subjects with both genotype data and either baseline only or with paired mammograms are listed in Table 1. Approximately one-third of subjects received prior tamoxifen, and about half of patients had received hormone replacement therapy (HRT) in the past.
Baseline mammographic density
Of the 385 subjects with evaluable baseline mammograms, the mean MPD assessed by the Reference Standard method was 17.8% (s.d. 11.1%; Table 1). Of the 259 subjects with both mammograms available for analysis, the mean baseline MPD was 17.1% (s.d. 10.7%). Of the 259 subjects, 82 (31.7%) had baseline MPD ⩾20%.
There was a statistically significant correlation between baseline MPD and age (P=0.038) and BMI (P<0.0001). Patients who had received prior chemotherapy had a higher baseline MPD (20.2% (s.d. 11.6%)) compared with those who had not (16.1% (s.d. 10.3%), P<0.0003). No statistically significant associations were detected with baseline E1S, prior tamoxifen, prior HRT, duration of prior tamoxifen or HRT, or time since discontinuation of tamoxifen or HRT (Table 2 and data not shown). Multivariate analysis revealed that BMI and prior chemotherapy remained statistically significantly associated with baseline MPD (Table 2).
There was also a statistically significant association between prior chemotherapy and baseline true dense area on both univariate and multivariate analyses. Patients who had received prior chemotherapy had a higher true dense area (3005 mm2 (s.d. 2019)) compared with those who had not (2547 mm2 (s.d. 1571), P=0.013). No other statistically significant associations were identified with any of the other factors listed in Table 2.
Change in breast density with AI therapy
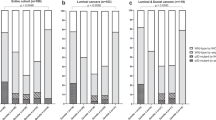
Of the 259 subjects with both baseline and 24-month mammograms and genotype data available, there was a statistically significant absolute mean decrease in MPD of 1.9% (s.d. 4.9%), which corresponds to an average percentage decrease of 5.6% (s.d. 34.7%; Table 3). Two-thirds of subjects (171 of 259) had a decrease in MPD with AI therapy (Figure 2). The baseline MPD of those subjects whose MPD decreased with AI therapy was 19.4% (s.d. 10.9%), whereas the baseline MPD of those subjects whose MPD was stable or increased was 12.6% (s.d. 8.8%). For those subjects with baseline MPD ⩾20%, the average absolute decrease in MPD with 2 years of AI therapy was 4.7% (s.d. 5.5%), whereas for those subjects with baseline MPD <20%, the average absolute decrease in MPD was 0.6% (s.d. 4.0%). This difference was statistically significant (P<0.00001).
Analysis of the baseline and 24-month mammograms in this clinical trial using BIRADS density classification, the standard method used clinically, demonstrated similar findings to those presented above using the computer-assessed Reference Standard method. As assessed using BIRADS density classification, 203 subjects (78.4%) had stable BIRADS, 47 (18.1%) experienced a decrease, and 9 (3.5%) experienced an increase (Supplementary Table S3). Using logistic regression, the decrease in BIRADS category with 24 months of AI therapy was statistically significantly associated with a greater baseline BIRADS category (β −0.22, P<0.0001).
In univariate analysis, there was a statistically significant association between absolute decrease in MPD and both higher baseline MPD (P<0.0001) and lower baseline BMI (P<0.002; Table 4). No association was detected between absolute change in MPD and age, baseline E1S, change in E1S, prior chemotherapy, prior tamoxifen, prior HRT, duration of prior tamoxifen or HRT, or time since discontinuation of tamoxifen or HRT (Table 4 and data not shown). The absolute change in MPD did not differ according to which AI the subjects received (exemestane −1.7%, letrozole −2.1%, P=0.95). On multivariate analysis, only baseline MPD remained statistically significant (Table 4).
Analysis of associations between absolute change in true dense area and the same factors listed above only identified a statistically significant association with baseline MPD, which was present on both univariate and multivariate analyses. In contrast to the association with change in MPD, there was no association identified between change in true dense area and baseline BMI (P=0.17; Table 4).
Associations between candidate genetic variants and breast density
After adjustment for multiple comparisons, no statistically significant associations were identified between inherited genetic variants in candidate genes and pretreatment (baseline) MPD or true dense area. Similarly, after correction for multiple comparisons, no SNPs in any candidate genes were significantly associated with absolute change in MPD because of treatment with either of the AIs for 2 years (n=259).
Discussion
In this prospective clinical trial, we demonstrated that treatment with 2 years of adjuvant AI therapy results in a statistically significant decrease in MPD. Importantly, we found that subjects with higher baseline MPD experienced a greater absolute reduction in MPD during therapy than those with lower baseline MPD using both the Reference Standard method and the standard BIRADS density classification method used in the clinic. In addition, in the total population, there were no detectable significant associations between absolute change in MPD and any of the candidate inherited genetic variants.
Our findings are similar to those previously reported by Prowell et al (2011) and Vachon et al (2013), both of whom reported small decreases in MPD with approximately a year of AI therapy. In two small substudies derived from the MAP.1 and MAP.2 prevention trials of AI therapy, no significant decrease in MPD was observed with 1 to 2 years of therapy (Cigler et al, 2010, 2011). Although we demonstrated a statistically significant absolute decrease in both MPD and true breast density during treatment, the magnitude of the decrease is comparable to what has been reported in postmenopausal women who are not receiving anti-oestrogen therapy (Freedman et al, 2001). Importantly, the average age of subjects in Freedman’s study was 52 years, which is 8 years younger than our cohort. Research has shown greater declines in breast density between the ages of 45 and 55 years, with less change in breast density at older ages (Maskarinec et al, 2006). Prior research has also demonstrated relatively stable MPD over time in postmenopausal women with a BMI >30 kg m−2 (Maskarinec et al, 2006). Therefore, the decrease in MPD over 2 years observed in this study may in fact be clinically relevant given the characteristics of the enrolled patient cohort.
The observation that those with higher baseline MPD had a greater decrease in MPD during AI therapy confirms a similar finding recently reported by Vachon et al (2013). In addition, this observation is clinically important, as others have demonstrated that (1) high MPD is associated with breast cancer risk (Boyd et al, 2005) and (2) a decrease in MPD is associated with a decreased risk of developing breast cancer and better breast cancer outcomes (Kerlikowske et al, 2007; Cuzick et al, 2011; Kim et al, 2012; Li et al, 2013). A population-based study of women without breast cancer revealed a decreased risk of breast cancer in women whose BIRADS density category was 2 or 3 at study entry, and which decreased over an average of 3 years (Kerlikowske et al, 2007). In a tamoxifen prevention study, Cuzick et al (2011) reported no statistically significant reduction in risk of breast cancer for those whose MPD decreased by <10%. Similarly, postmenopausal women with breast cancer who experienced a relative reduction in MPD of >20% during adjuvant tamoxifen therapy had a 50% decrease in the risk of breast cancer mortality (Li et al, 2013). Finally, in a retrospective study of women with nonmetastatic breast cancer, Kim et al (2012) reported a trend towards an association between a lesser decrease in MPD with AI therapy and increased risk of disease recurrence (hazard ratio 7.11 (95% CI 0.90–56.37), P=0.06). However, the patients in the study of Kim et al (2012) were younger and only 16% were treated with AI monotherapy. Therefore, the applicability of these findings to a postmenopausal population of breast cancer survivors treated with upfront AI therapy remains uncertain.
Importantly, only two-thirds of patients in our study experienced a decrease in MPD with AI therapy. It remains unclear why the other one-third of patients had an increase in MPD with therapy (Figure 2). One possible explanation for the lack of a decrease is the low MPD in these subjects at baseline (mean 12.6%), which may limit further decreases in MPD. However, the 15 (5.8%) subjects who experienced an absolute increase in MPD of >5% did not all have low MPD before treatment initiation, as the median baseline value was 12.9%, and ranged from 4.7 to 35.3%. This finding is concerning clinically given the findings of worse breast cancer outcomes or no breast cancer risk reduction in patients who experienced less reduction in MPD with endocrine therapy, as described above (Cuzick et al, 2011; Kim et al, 2012; Li et al, 2013). Similarly, increasing breast density over 3 years in women without a history of breast cancer has been associated with an increased risk of breast cancer (Kerlikowske et al, 2007). Therefore, the possibility that an increase in MPD with AI therapy portends a poor prognosis is deserving of further study.
In multivariate analysis, we demonstrated correlations between lower baseline MPD and both higher BMI and prior treatment with chemotherapy, which are consistent with previously reported findings (Vachon et al, 2000; Kim et al, 2012). We also demonstrated correlations between greater decrease in MPD and higher baseline MPD, which was also consistent with previously reported findings by Kim et al (2012) but inconsistent with the MAP prevention trials (Cigler et al, 2010, 2011). We failed to detect any associations between baseline MPD and either age or prior tamoxifen use in our postmenopausal cohort.
The strengths of this study include that it was a relatively large prospective trial, and that subjects were randomly assigned to treatment with one of two different AIs from different classes (steroidal vs nonsteroidal). In addition, the method used to determine MPD has previously been validated (Martin et al, 2006), including use in a study evaluating genetic associations with breast density (Douglas et al, 2008), and had multiple prospective quality measures in place to minimise intraobserver variability and other sources of bias as described in the Materials and Methods section. Importantly, we analysed the raw data files rather than processed digital images for all digital mammograms. As data processing methods can differ between manufacturer and over time, care was taken not to introduce variability into the mammogram findings. This issue must be considered when evaluating results of studies that analysed mammogram data using clinical databases, which typically store only processed images.
A potential limitation of this study, as has been noted in other studies of AI therapy, is the large treatment discontinuation rate due primarily to treatment-emergent toxicity (Henry et al, 2012). It is unknown whether the development of side effects could be associated with unidentified factors that also affect change in breast density. However, our results are similar to those from Prowell et al (2011), in which no subjects discontinued therapy, suggesting that the high rate of treatment discontinuation may not have had a great impact. Another limitation is the unavoidable lack of an untreated group, as it is uncertain how much of a decrease in MPD related to ageing would have been seen in the absence of AI therapy.
Review of the literature reveals a large number of previously reported clinical studies that have also failed to observe consistent associations between SNPs and breast density in either healthy women or patients with breast cancer (Haiman et al, 2002, 2003; Hong et al, 2003, 2004; Warren et al, 2006; Olson et al, 2007; Li et al, 2010; Lindstrom et al, 2011). Few studies have evaluated associations between change in MPD with endocrine therapy and inherited variants. Consistent with the previously reported studies, we did not identify any statistically significant associations between inherited genetic variants and baseline MPD. In addition, we were unable to identify any associations between inherited variants and change in MPD with AI therapy, although, as described above, the statistical power for this analysis was limited because of the high rate of treatment discontinuation.
In summary, we report a decrease in mammographic breast density with 2 years of AI therapy in the majority of treated patients, with a greater decrease in those with higher MPD at baseline. This finding has potential clinical implications, as other studies have reported associations between greater reductions in MPD during endocrine therapy and better breast cancer outcomes (Cuzick et al, 2011; Kim et al, 2012). It remains unknown whether change in MPD with AI therapy may be a useful early marker for monitoring effectiveness of adjuvant treatment.
Change history
29 October 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Aiello EJ, Tworoger SS, Yasui Y, Stanczyk FZ, Potter J, Ulrich CM, Irwin M, McTiernan A (2005) Associations among circulating sex hormones, insulin-like growth factor, lipids, and mammographic density in postmenopausal women. Cancer Epidemiol Biomarkers Prev 14: 1411–1417.
American College of Radiology (2003) American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) 4th edn. American College of Radiology Reston, VA.
Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ (1995) Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst 87: 670–675.
Boyd NF, Dite GS, Stone J, Gunasekara A, English DR, McCredie MR, Giles GG, Tritchler D, Chiarelli A, Yaffe MJ, Hopper JL (2002) Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med 347: 886–894.
Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ (1998) Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev 7: 1133–1144.
Boyd NF, Martin LJ, Li Q, Sun L, Chiarelli AM, Hislop G, Yaffe MJ, Minkin S (2006) Mammographic density as a surrogate marker for the effects of hormone therapy on risk of breast cancer. Cancer Epidemiol Biomarkers Prev 15: 961–966.
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD (2005) Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol 6: 798–808.
Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ (2010) American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28: 3784–3796.
Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ (1994) The quantitative analysis of mammographic densities. Phys Med Biol 39: 1629–1638.
Cigler T, Richardson H, Yaffe MJ, Fabian CJ, Johnston D, Ingle JN, Nassif E, Brunner RL, Wood ME, Pater JL, Hu H, Qi S, Tu D, Goss PE (2011) A randomized, placebo-controlled trial (NCIC CTG MAP.2) examining the effects of exemestane on mammographic breast density, bone density, markers of bone metabolism and serum lipid levels in postmenopausal women. Breast Cancer Res Treat 126: 453–461.
Cigler T, Tu D, Yaffe MJ, Findlay B, Verma S, Johnston D, Richardson H, Hu H, Qi S, Goss PE (2010) A randomized, placebo-controlled trial (NCIC CTG MAP1) examining the effects of letrozole on mammographic breast density and other end organs in postmenopausal women. Breast Cancer Res Treat 120: 427–435.
Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM (2011) Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst 103: 744–752.
Cuzick J, Warwick J, Pinney E, Warren RM, Duffy SW (2004) Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst 96: 621–628.
Desta Z, Kreutz Y, Nguyen AT, Li L, Skaar T, Kamdem LK, Henry NL, Hayes DF, Storniolo AM, Stearns V, Hoffmann E, Tyndale RF, Flockhart DA (2011) Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin Pharmacol Ther 90: 693–700.
Douglas JA, Roy-Gagnon MH, Zhou C, Mitchell BD, Shuldiner AR, Chan HP, Helvie MA (2008) Mammographic breast density—evidence for genetic correlations with established breast cancer risk factors. Cancer Epidemiol Biomarkers Prev 17: 3509–3516.
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28: 509–518.
Ellingjord-Dale M, Lee E, Couto E, Ozhand A, Qureshi SA, Hofvind S, Van Den Berg DJ, Akslen LA, Grotmol T, Ursin G (2012) Polymorphisms in hormone metabolism and growth factor genes and mammographic density in Norwegian postmenopausal hormone therapy users and non-users. Breast Cancer Res 14: R135.
Freedman M, San Martin J, O’Gorman J, Eckert S, Lippman ME, Lo SC, Walls EL, Zeng J (2001) Digitized mammography: a clinical trial of postmenopausal women randomly assigned to receive raloxifene, estrogen, or placebo. J Natl Cancer Inst 93: 51–56.
Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H (2011) Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med 364: 2381–2391.
Greendale GA, Palla SL, Ursin G, Laughlin GA, Crandall C, Pike MC, Reboussin BA (2005) The association of endogenous sex steroids and sex steroid binding proteins with mammographic density: results from the Postmenopausal Estrogen/Progestin Interventions Mammographic Density Study. Am J Epidemiol 162: 826–834.
Greenwood CM, Paterson AD, Linton L, Andrulis IL, Apicella C, Dimitromanolakis A, Kriukov V, Martin LJ, Salleh A, Samiltchuk E, Parekh RV, Southey MC, John EM, Hopper JL, Boyd NF, Rommens JM (2011) A genome-wide linkage study of mammographic density, a risk factor for breast cancer. Breast Cancer Res 13: R132.
Haiman CA, Bernstein L, Berg D, Ingles SA, Salane M, Ursin G (2002) Genetic determinants of mammographic density. Breast Cancer Res 4: R5.
Haiman CA, Hankinson SE, De Vivo I, Guillemette C, Ishibe N, Hunter DJ, Byrne C (2003) Polymorphisms in steroid hormone pathway genes and mammographic density. Breast Cancer Res Treat 77: 27–36.
Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, Stearns V, Hayes DF, Storniolo AM (2012) Predictors of aromatase inhibitor discontinuation due to treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30: 936–942.
Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, Hayden J, Lemler S, Shahverdi K, Powers P, Li L, Flockhart D, Stearns V, Hayes DF, Storniolo AM, Clauw DJ (2008) Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat 111: 365–372.
Hong CC, Thompson HJ, Jiang C, Hammond GL, Tritchler D, Yaffe M, Boyd NF (2003) Val158Met polymorphism in catechol-O-methyltransferase gene associated with risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev 12: 838–847.
Hong CC, Thompson HJ, Jiang C, Hammond GL, Tritchler D, Yaffe M, Boyd NF (2004) Association between the T27C polymorphism in the cytochrome P450 c17alpha (CYP17) gene and risk factors for breast cancer. Breast Cancer Res Treat 88: 217–230.
Kerlikowske K, Ichikawa L, Miglioretti DL, Buist DS, Vacek PM, Smith-Bindman R, Yankaskas B, Carney PA, Ballard-Barbash R (2007) Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst 99: 386–395.
Kim J, Han W, Moon HG, Ahn SK, Shin HC, You JM, Han SW, Im SA, Kim TY, Koo HR, Chang JM, Cho N, Moon WK, Noh DY (2012) Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res 14: R102.
Li J, Eriksson L, Humphreys K, Czene K, Liu J, Tamimi RM, Lindstrom S, Hunter DJ, Vachon CM, Couch FJ, Scott CG, Lagiou P, Hall P (2010) Genetic variation in the estrogen metabolic pathway and mammographic density as an intermediate phenotype of breast cancer. Breast Cancer Res 12: R19.
Li J, Humphreys K, Eriksson L, Edgren G, Czene K, Hall P (2013) Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal breast cancer patients. J Clin Oncol 31: 2249–2256.
Lindstrom S, Vachon CM, Li J, Varghese J, Thompson D, Warren R, Brown J, Leyland J, Audley T, Wareham NJ, Loos RJ, Paterson AD, Rommens J, Waggott D, Martin LJ, Scott CG, Pankratz VS, Hankinson SE, Hazra A, Hunter DJ, Hopper JL, Southey MC, Chanock SJ, Silva Idos S, Liu J, Eriksson L, Couch FJ, Stone J, Apicella C, Czene K, Kraft P, Hall P, Easton DF, Boyd NF, Tamimi RM (2011) Common variants in ZNF365 are associated with both mammographic density and breast cancer risk. Nat Genet 43: 185–187.
Martin KE, Helvie MA, Zhou C, Roubidoux MA, Bailey JE, Paramagul C, Blane CE, Klein KA, Sonnad SS, Chan HP (2006) Mammographic density measured with quantitative computer-aided method: comparison with radiologists’ estimates and BI-RADS categories. Radiology 240: 656–665.
Martin LJ, Boyd NF (2008) Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res 10: 201.
Maskarinec G, Pagano I, Lurie G, Kolonel LN (2006) A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 15: 732–739.
Olson JE, Ma CX, Pelleymounter LL, Schaid DJ, Pankratz VS, Vierkant RA, Fredericksen ZS, Ingle JN, Wu Y, Couch F, Sellers TA, Weinshilboum RM, Vachon CM (2007) A comprehensive examination of CYP19 variation and breast density. Cancer Epidemiol Biomarkers Prev 16: 623–625.
Pinsky RW, Helvie MA (2010) Mammographic breast density: effect on imaging and breast cancer risk. J Natl Compr Canc Netw 8: 1157–1164.
Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, Conant EF, Fajardo LL, Bassett L, D’Orsi C, Jong R, Rebner M (2005) Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 353: 1773–1783.
Prowell TM, Blackford AL, Byrne C, Khouri NF, Dowsett M, Folkerd E, Tarpinian KS, Powers PP, Wright LA, Donehower MG, Jeter SC, Armstrong DK, Emens LA, Fetting JH, Wolff AC, Garrett-Mayer E, Skaar TC, Davidson NE, Stearns V (2011) Changes in breast density and circulating estrogens in postmenopausal women receiving adjuvant anastrozole. Cancer Prev Res (Phila) 4: 1993–2001.
Rutter CM, Mandelson MT, Laya MB, Seger DJ, Taplin S (2001) Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA 285: 171–176.
Tamimi RM, Hankinson SE, Colditz GA, Byrne C (2005) Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev 14: 2641–2647.
Ursin G, Lillie EO, Lee E, Cockburn M, Schork NJ, Cozen W, Parisky YR, Hamilton AS, Astrahan MA, Mack T (2009) The relative importance of genetics and environment on mammographic density. Cancer Epidemiol Biomarkers Prev 18: 102–112.
Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA (2000) Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control 11: 653–662.
Vachon CM, Suman VJ, Brandt KR, Kosel ML, Buzdar AU, Olson JE, Wu FF, Flickinger LM, Ursin G, Elliott CR, Shepherd L, Weinshilboum RM, Goss PE, Ingle JN (2013) Mammographic breast density response to aromatase inhibition. Clin Cancer Res 19: 2144–2153.
Varghese JS, Smith PL, Folkerd E, Brown J, Leyland J, Audley T, Warren RM, Dowsett M, Easton DF, Thompson DJ (2012) The heritability of mammographic breast density and circulating sex-hormone levels; two independent breast cancer risk factors. Cancer Epidemiol Biomarkers Prev 72: 1478–1484.
Warren R, Skinner J, Sala E, Denton E, Dowsett M, Folkerd E, Healey CS, Dunning A, Doody D, Ponder B, Luben RN, Day NE, Easton D (2006) Associations among mammographic density, circulating sex hormones, and polymorphisms in sex hormone metabolism genes in postmenopausal women. Cancer Epidemiol Biomarkers Prev 15: 1502–1508.
Wei J, Chan HP, Helvie MA, Roubidoux MA, Sahiner B, Hadjiiski LM, Zhou C, Paquerault S, Chenevert T, Goodsitt MM (2004) Correlation between mammographic density and volumetric fibroglandular tissue estimated on breast MR images. Med Phys 31: 933–942.
Zhou C, Chan HP, Petrick N, Helvie MA, Goodsitt MM, Sahiner B, Hadjiiski LM (2001) Computerized image analysis: estimation of breast density on mammograms. Med Phys 28: 1056–1069.
Acknowledgements
This study was supported in part by Pharmacogenetics Research Network Grant no. U-01 GM61373 (to DAF) and Clinical Pharmacology training Grant 5T32-GM08425 (to DAF) from the National Institute of General Medical Sciences, National Institutes of Health, Bethesda, MD, and by Grant Numbers M01-RR000042 (UM), M01-RR00750 (IU), and M01-RR00052 (JHU) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. In addition, these studies were supported by grants from Pfizer, Inc. (to DFH), Novartis Pharma AG (to DFH), and the Fashion Footwear Association of New York/QVC Presents Shoes on Sale (to DFH). Study medication was provided by Pfizer (exemestane) and Novartis (letrozole). NLH receives research funding from AstraZeneca, Eli Lilly, sanofi Aventis, BiPar Pharmaceuticals, and Exelixis. JMR received a research grant from Pfizer. AMS receives research funding from Novartis and Pfizer. DAF receives research funding from Novartis and Pfizer. DFH receives research funding from AstraZeneca, Novartis, Pfizer, Veridex, and Janssen. VS receives research funding from Abraxis (Celegene), Merck, Novartis, and Pfizer. MAH receives research funding from General Electric Healthcare. We thank the participating patients and research study staff at each of the clinical trial sites.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies this paper on British Journal of Cancer website
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Henry, N., Chan, HP., Dantzer, J. et al. Aromatase inhibitor-induced modulation of breast density: clinical and genetic effects. Br J Cancer 109, 2331–2339 (2013). https://doi.org/10.1038/bjc.2013.587
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.587
Keywords
This article is cited by
-
Genetic architecture of mammographic density as a risk factor for breast cancer: a systematic review
Clinical and Translational Oncology (2023)
-
Identifying women with increased risk of breast cancer and implementing risk-reducing strategies and supplemental imaging
Breast Cancer (2022)
-
Effects of exemestane and letrozole therapy on plasma concentrations of estrogens in a randomized trial of postmenopausal women with breast cancer
Breast Cancer Research and Treatment (2017)
-
Prospective assessment of patient-reported outcomes and estradiol and drug concentrations in patients experiencing toxicity from adjuvant aromatase inhibitors
Breast Cancer Research and Treatment (2017)
-
Variable aromatase inhibitor plasma concentrations do not correlate with circulating estrogen concentrations in post-menopausal breast cancer patients
Breast Cancer Research and Treatment (2017)