Abstract
Background:
Pathologic complete response (pCR) to neoadjuvant treatment (NAT) is associated with improved survival of patients with HER2+ breast cancer. We investigated the ability of interim positron emission tomography (PET) regarding early prediction of pathology outcomes.
Methods:
During 61 months, consecutive patients with locally advanced or large HER2+ breast cancer patients without distant metastases were included. All patients received NAT with four cycles of epirubicin+cyclophosphamide, followed by four cycles of docetaxel+trastuzumab. 18F-fluorodeoxyglucose (18F-FDG)-PET/computed tomography (CT) was performed at baseline (PET1) and after two cycles of chemotherapy (PET2). Maximum standardised uptake values were measured in the primary tumour as well as in the axillary lymph nodes. The correlation between pathologic response and SUV parameters (SUVmax at PET1, PET2 and ΔSUVmax) was examined with the t-test. The predictive performance regarding the identification of non-responders was evaluated using receiver operating characteristics (ROC) analysis.
Results:
Thirty women were prospectively included and 60 PET/CT examination performed. At baseline, 22 patients had PET+ axilla and in nine of them 18F-FDG uptake was higher than in the primary tumour. At surgery, 14 patients (47%) showed residual tumour (non-pCR), whereas 16 (53%) reached pCR. Best prediction was obtained when considering the absolute residual SUVmax value at PET2 (AUC=0.91) vs 0.67 for SUVmax at PET1 and 0.86 for ΔSUVmax. The risk of non-pCR was 92.3% in patients with any site of residual uptake >3 at PET2, no matter whether in breast or axilla, vs 11.8% in patients with uptake ⩽3 (P=0.0001). The sensitivity, specificity, PPV, NPV and overall accuracy of this cutoff were, respectively: 85.7%, 93.8%, 92.3%, 88.2% and 90%.
Conclusion:
The level of residual 18F-FDG uptake after two cycles of chemotherapy predicts residual disease at completion of NAT with chemotherapy+trastuzumab with high accuracy. Because many innovative therapeutic strategies are now available (e.g., addition of a second HER2-directed therapy or an antiangiogenic), early prediction of poor response is critical.
Similar content being viewed by others
Main
Neoadjuvant chemotherapy was initially developed for primary inoperable breast cancer, and is now also widely used in operable but large breast cancer not eligible to breast-conserving therapy (NCCN Guidelines, 2013). Positron emission tomography/computed tomography with 18F-fluorodeoxyglucose (18F-FDG-PET/CT) is a useful staging modality in these patients (Fuster et al, 2008; Groheux et al, 2012a, 2013a). Moreover, some studies have demonstrated a correlation between early changes in 18F-FDG primary tumour uptake after one or two courses of chemotherapy and the extent of pathology response at completion of treatment, at the tumour level (Schwarz-Dose et al, 2009; Wang et al, 2012), as well as in axillary lymph nodes (Straver et al, 2010; Rousseau et al, 2011). However, the ability to implement 18F-FDG-PET/CT as a surrogate marker for treatment efficacy remains unclear because of substantial heterogeneities across studies and also because breast cancer cannot be examined as a single entity (Groheux et al, 2011a, 2012b, 2013b; Humbert et al, 2012). Breast cancer comprises different phenotypes with different response rates to chemotherapy, different treatment options and different prognoses (NCCN Guidelines, 2013). We therefore suggested that the clinical aims of early 18F-FDG monitoring and the criteria used to predict efficacy should be determined in specific subgroups (Groheux et al, 2011a, 2012b, 2013b).
Overexpression of the HER2 receptor occurs in roughly 20% of breast tumours. The prognosis of this aggressive entity has been improved with the advent of trastuzumab therapy, an antibody targeting the HER2 receptor (Gianni et al, 2010). In the neoadjuvant setting, pathologic complete response (pCR) at surgery is higher with the addition of trastuzumab and is correlated with improved outcomes, suggesting that it may serve as a surrogate marker of clinical benefit (Gianni et al, 2010; Untch et al, 2011; Von Minckwitz et al, 2012). Identifying poor responders before completion of neoadjuvant treatment (NAT) might be useful for improving outcome by allowing an early switch to a different chemotherapy regimen, and/or the use of more than one targeted therapy (Baselga et al, 2012; Gianni et al, 2012; Guarneri et al, 2012; Pierga et al, 2012). The objective of this prospective study was to assess the value of interim 18F-FDG-PET/CT for early identification of HER2+ breast cancer patients who will not achieve pCR with a conventional chemotherapy/trastuzumab NAT.
Materials and methods
Patients
During 61 months, patients with clinical stage II or III HER2+ breast cancer seen at the breast disease unit of Saint Louis hospital, and scheduled for NAT were included. All patients underwent an 18F-FDG-PET/CT scan at baseline (PET1) and another scan after two cycles of chemotherapy (PET2). Patients with distant metastases identified at initial staging were not included, because these patients receive treatments tailored to metastatic state and patient characteristics. After completion of NAT, all patients underwent surgery and response to treatment was assessed by pathology examination of surgical specimens. The study followed the guidelines of the institutional ethical committee with informed patient consent.
Neoadjuvant treatment
All patients received four cycles of epirubicin (75 mg m−2) plus cyclophosphamide (750 mg m−2) administered every 3 weeks, followed by four courses of docetaxel (100 mg m−2 every 3 weeks) plus trastuzumab (8 mg kg−1 loading dose, followed by 6 mg kg−1 every 3 weeks).
18F-FDG-PET/CT imaging
Patients fasted for 6 h. Blood glucose level had to be <7 mmol l−1. 18F-fluorodeoxyglucose (5 MBq kg−1; not exceeding 500 MBq) was administered 60 min before data were acquired on a Gemini XL PET/CT scanner (Philips Healthcare, Eindhoven, The Netherlands). Computed tomography was acquired first (120 kV; 100mAs; no contrast enhancement). Positron emission tomography three-dimensional (3D) data were acquired in a list-mode format with 2 min per bed position, and images were reconstructed using a 3D row-action maximum-likelihood algorithm.
Positron emission tomography/CT images were interpreted by two nuclear medicine specialists blinded to the patient’s record. 18F-fluorodeoxyglucose uptake was expressed as SUV (standardised uptake value). A 3D region of interest (3D-ROI) was drawn around the primary tumour and around axillary lymph nodes when present. Maximum SUV value within each ROI was recorded and used for the study analysis. The lesion with the highest initial uptake (either the primary tumour or an axillary lymph node) was considered the main ‘target’ to assess. The change in SUVmax after two cycles of chemotherapy was expressed as follows:

Tumour histology and immunohistochemistry analysis
Breast cancer diagnosis was performed on a core-needle biopsy. Tumour grade was determined using the modified Scarff–Bloom–Richardson classification. Tumours were classified as oestrogen receptor positive (ER+) if showing moderate or high positivity (2 or 3+) of at least 10% of cells. The same criteria were used for progesterone receptor (PR) status. Tumours were classified as HER2 overexpression (HER2+) if IHC staining results were 3+ (uniform, intense membrane staining of >30% of invasive tumour cells). Equivocal results (2+) were further tested by FISH or SISH (Wolff et al, 2007).
Pathology assessment at completion of NAT
Pathologic complete response (pCR) was defined as no evidence of residual invasive cancer, both in breast tissue and lymph nodes. Absence of in situ carcinoma was not required to define pCR.
Statistical analysis
Statistical analyses were performed using Medcalc (MedCalc Software, Ostend, Belgium). P<0.05 was considered statistically significant. Maximum standardised uptake values were measured in the primary tumour as well as in the axillary lymph nodes. The correlation between pathologic response and SUV parameters (SUVmax at baseline, at two cycles and ΔSUVmax) was examined with the t-test and log-transformed data because of non-normality of the distributions. The predictive performance regarding the identification of non-responders was evaluated using ROC analysis. Correlations between clinical or biological parameters (axillary status, tumour grade, ER expression) and pathologic response were assessed with the t-test. The influence of biological parameters on SUVmax value of primary tumour on baseline PET study was also assessed with the t-test.
Results
Tumours characteristics and pathological response
Thirty consecutive patients with large or locally advanced HER2+ breast cancer and no distant metastases at baseline staging were included (Table 1). Eight patients could not be included because distant metastases were discovered on baseline 18F-FDG imaging.
All patients received the eight courses of NAT. At completion of NAT, 14 patients received breast-conserving surgery and the others mastectomy. All patients had axillary lymph node dissection. Histopathology showed residual invasive disease (non-pCR) in 14 (47%), whereas 16 patients (53%) had pCR with no residual invasive disease in axilla and in breast (in situ carcinoma was present in three of them).
Absolute value of SUVmax at PET1 and PET2
Individual PET data and pathological response are outlined in Table 2 and associations between PET parameters with response at completion of chemotherapy are shown in Table 3.
At PET1, SUVmax of breast tumour ranged between 2.4 and 18.8 (mean=7.9). Twenty-two patients had PET+ axilla (SUVmax: 1.9–23.9; mean=8.2), and in nine of them 18F-FDG uptake was higher than in the primary tumour.
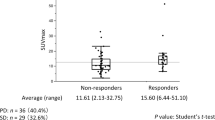
Maximum standardised uptake value of the site with highest uptake (target lesion) at PET1 ranged between 2.4 and 23.9 (mean=9.4). There was some, nonsignificant, correlation between high baseline SUVmax of the target lesion and residual disease at completion of NAT (P=0.08) (Figure 1).
Distributions of absolute values of SUVmax at PET1 (A ) and PET2 (B ) of the site with highest uptake (breast primary cancer or axillary node) and ΔSUVmax values ( C ), for non-pCR and pCR patients; the optimal cutoff values of SUVmax (PET1)=9.1, SUVmax (PET2)=3 and ΔSUVmax=−62% are drawn on the graphs (dashed lines).
At PET2, breast tumour SUVmax ranged between 1.1 and 14.1 (mean=4.0) and SUVmax in lymph nodes ranged between 0.6 and 24.1 (mean=3.4).
There was a strong correlation between the highest residual 18F-FDG uptake at PET2 and pathological response (mean residual SUVmax=7.7 in non-pCR vs 2.1 in pCR patients, P=0.0001; Table 3). Best prediction was obtained with a cutoff of SUVmax=3 (Figure 1), which allowed identification of poor responders (non-pCR) with a sensitivity, specificity, PPV, NPV and overall accuracy of, respectively: 85.7% (12 out of 14), 93.8% (15 out of 16), 92.3% (12 out of 13), 88.2% (15 out of 17) and 90% (27 out of 30).
The risk of non-pCR was 92.3% in patients with any site of residual uptake >3 on interim PET, no matter whether in breast or axilla, vs 11.8% in patients with uptake ⩽3 (P=0.0001).
The accuracy of PET2 SUVmax was even higher (95.5%; 21 out of 22) in the subset of patients with positive nodes on baseline PET (i.e., those with more than one 18F-FDG uptake site).
Evolution of SUVmax between PET1 and PET2
The mean ΔSUVmax for the target lesion (site of highest initial uptake, either breast primary or axillary node) was −51% (range: −95 to +24%) (Table 3). The mean decrease in SUVmax was significantly larger in patients who achieved pCR (−66±18%) than in those who did not (−33±30%) (P=0.001) (Figure 1). Using a cutoff of <62% decrease in SUVmax, residual disease (non-pCR) was depicted with a sensitivity of 85.7%, a specificity of 75%, and an overall accuracy of 80% (24 out of 30).
Prediction based on ΔSUVmax was more modest when considering only the primary tumour. In this case, the cutoff of 62% decrease in SUVmax predicted pathology outcomes with an overall accuracy of 73.3% (22 out of 30) (sensitivity 85.7%, specificity 62.5%).
Optimal PET parameter for prediction (ROC analysis)
Figure 2 shows the AUC for prediction of pathology outcomes for each of the following PET parameters: SUVmax value of target lesion at PET1; SUVmax value of target lesion at PET2; and ΔSUVmax of the target lesion. Absolute SUVmax at PET2 offers the highest performance (AUC=0.91 vs 0.67 for SUVmax at PET1 and 0.86 for ΔSUVmax). This is also the case when examining the subset of 22 patients with initially node-positive axilla (AUC=0.99 vs 0.73 for SUVmax at PET1 and 0.89 for ΔSUVmax).
Areas under the receiver operator curves (AUCs) showing the ability of different PET parameters at predicting residual disease (non-pCR) for the 30 HER2+ breast cancer women ( A ): SUVmax value of target lesion at PET1 (green curve); SUVmax value of target lesion at PET2 (blue curve); and ΔSUVmax of the target lesion (orange curve).The absolute SUVmax value at PET2 provides the highest performance (AUC=0.91). Similar curves are drawn for the subset of 22 patients with baseline node-positive axilla (B). Again, best prediction is offered by the absolute SUVmax value at PET2 (AUC=0.99). The full colour version of this figure is available at British Journal of Cancer online.
Figures 3 and 4 show initial and interim PET/CT images in two patients, a patient who achieved pCR (Figure 3) and one who did not (Figure 4).
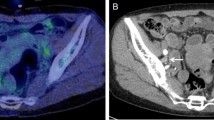
A 48-year-old woman (patient no. 10; Table 2 ) with HER2-overexpressing tumour of left breast. Positron emission tomography and PET/CT fusion images of the primary tumour and axillary lymph nodes, at baseline (A–D), and after two courses of chemotherapy (E–H). At baseline, SUVmax was 6.2 in breast tumour (A and B) and 6.7 in a lymph node (C and D). At PET2, SUVmax was 1.3 in breast tumour (arrow, E and F) (ΔSUV=−79%) and 0.9 in the lymph node (arrow, G and H) (ΔSUV=−87%). At completion of NAT, breast and axillary surgery showed ‘pCR’.
A 49-year-old woman (patient no. 20; Table 2 ) with HER2-overexpressing tumour of right breast. Positron emission tomography and PET/CT fusion images of the primary tumour (A and B) and an axillary lymph node (C and D) at baseline and corresponding images after two courses of chemotherapy (E–H). At baseline SUVmax was 11 in breast tumour (arrow, A and B) and 16.2 in axillary lymph node (target lesion) (arrow, C and D). At PET2, SUVmax was 5.9 in breast tumour (arrow, E and F) (ΔSUV=−46%), and 13.4 in the lymph node (arrow, G and H) (ΔSUV=−17%). At completion of NAT, surgery showed residual invasive tumour.
Impact of clinical and biological parameters
Baseline SUVmax values were significantly higher in grade 3 than in grade 2 primary breast tumours (mean: 9.0±4.8 vs 5.5±2.7; P<0.03), corroborating our previous findings in a general population of breast cancer (Groheux et al, 2011b). However, in these patients with HER2 overexpression, there was no significant difference in baseline SUVmax values between ER+ and ER− tumours (mean: 8.3±4.9 vs 7.7±4.4; P=0.7).
Neither initial axillary status (N+ vs N0) (P=0.3) nor ER status (P=0.8), or tumour grade (P=0.5), showed statistically significant association with pathological response.
Discussion
In the present series of patients with large or locally advanced HER2+ non-metastatic breast cancer who received NAT with four cycles of epirubicin+cyclophosphamide, followed by four cycles of docetaxel+trastuzumab, pCR was achieved in 53% of patients, whereas 47% had residual tumour at surgery, which is in agreement with other reports (Untch et al, 2011; Humbert et al, 2012).
We examined the ability of 18F-FDG-PET/CT in predicting pathology outcomes after only two cycles of chemotherapy. The lesion with the baseline highest 18F-FDG uptake was considered the target lesion for assessing the change in uptake under therapy (ΔSUV). Axilla was thus the target in nine patients in whom 18F-FDG uptake in axillary nodes was higher than in the primary tumour (Table 2). This refinement offered a modest improvement in PET prediction based on ΔSUV. Using 62% decrease in SUVmax at two cycles as cutoff, pathology findings were predicted with an overall accuracy of 80% (24 out of 30), whereas analysis taking into consideration the primary tumour alone offered a lower accuracy of 73.3% (22 out of 30). This accuracy offered by ΔSUV was similar to that (76%) reported by Humbert et al (2012). Koolen et al (2013) found lower predictive value of ΔSUV in HER2+ patients.
However, as evidenced in the present series, ΔSUV is probably not the appropriate PET parameter for assessing response in this breast cancer phenotype. The absolute value of residual 18F-FDG uptake on interim PET, whatever the site of residual uptake (breast or axilla), was the single most important parameter in the prediction of pathology outcome (Figures 1 and 2). Any site of residual 18F-FDG uptake with an SUVmax >3 was predictive of non-pCR with a sensitivity of 85.7%, a specificity of 93.8% and an overall accuracy of 90% (27 out of 30). Accuracy was even higher (95.5%) when considering the 22 patients who had 18F-FDG-avid node on baseline PET.
These findings are in contrast with those we obtained in patients with triple-negative phenotype, in whom ΔSUV was the important determinant (Groheux et al, 2012b), further confirming our thought that each breast cancer phenotype should be examined as a separate entity when assessing the value of 18F-FDG-PET for monitoring response to treatment (Groheux et al, 2011a).
Among PET parameters, other than SUVmax, metabolic tumour volume (MTV) and total lesion glycolysis (TLG) are potentially useful parameters. In a previous pilot series, we have compared the value of various PET image-derived indices in predicting response to therapy in locally advanced breast cancer. We found that TLG had better predictive value than SUVmax for the subgroup of luminal tumours (RH+/HER2−), but not in triple-negative breast cancer or HER2+ breast cancer (Hatt et al, 2013). In the present study that deals with the HER2+ subtype we therefore did not include MTV or TLG parameters.
Any study investigating sequential PET scans needs to ensure the highest reproducibility possible regarding the acquisition protocol (Boellaard et al, 2010). In this study, imaging conditions were optimised to reduce the variability of the acquisition to a minimum.
Our findings show that the quality of early response to chemotherapy is an important determinant of the final outcome of a sequential treatment combining an anthracycline-based initial regimen (here epirubicin+cyclophosphamide), followed by trastuzumab+a taxane (here docetaxel). Such regimen is one of the more widely used (NCCN Guidelines, 2013). As stated in the National Comprehensive Cancer Network guidelines, ‘Retrospective evidence suggests that anthracycline-based chemotherapy regimens may be superior to non-anthracycline-based regimens in patients with HER2+ tumours’ (NCCN Guidelines, 2013). Trastuzumab is given sequentially and not concurrently with anthracyclines because of cardiac toxicity (NCCN Guidelines, 2013). Also, there is a synergistic activity of trastuzumab and docetaxel (Arnould et al, 2006).
There are many other neoadjuvant schemes in HER2+ patients. In a study of 37 patients treated from the outset with trastuzumab+docetaxel (±carboplatin), the average ΔSUVmax after one course was −71% in pCR vs −47% in non-pCR cases (P=0.01) (Humbert et al, 2012). A cutoff of ΔSUVmax of −75% offered an accuracy of 76% in predicting pathology outcome (Humbert et al, 2012). On the basis of these findings, the authors planned a randomised trial to assess the benefit of adding bevacizumab when the decrease in SUVmax is <70% after the first course of docetaxel+trastuzumab (Cochet et al, 2012). Prediction under anti-HER2 therapy alone is also possible, although the extent of decrease in SUVmax might be more modest. In a report on a sequential schema where the authors introduced first anti-HER2-therapy alone for 6 weeks (either trastuzumab, or lapatinib, or their combination), 60% of patients showed a metabolic response (decrease in SUV >25%; EORTC criteria). The pCR rate was two times as high in PET ‘responders’ compared with ‘non-responders’ (44% vs 19%, P=0.05) (Baselga et al, 2012; Gebhart et al, 2012). In all these studies, assessing the efficacy of PET prediction after having introduced trastuzumab, the accuracy of interim PET was rather modest, and lower than in the present series (90%). One explanation could be that the decrease in 18F-FDG uptake under trastuzumab is not a pure reflect of cell killing but also reflects other specific impact on glucose metabolism, such as reduction in Glut1 and in Hexokinase II activity, as evidenced in animal studies with tumour xenografts (Smith et al, 2013). False positives might also result from inflammatory response induced by trastuzumab, as hypothesised by Koolen et al (2013).
In patients with HER2+ tumour, pCR is a powerful predictor of clinical outcome (Untch et al, 2011; Von Minckwitz et al, 2012). Early detection with interim PET of patients who are unlikely to achieve pCR should therefore be helpful to adapt treatment. Several recent works suggest that dual inhibition of HER2 (trastuzumab+lapatinib or trastuzumab+pertuzumab) has high efficacy (Baselga et al, 2012; Gianni et al, 2012; Guarneri et al, 2012). Another approach used bevacizumab together with trastuzumab and chemotherapy (Pierga et al, 2012). However, these strategies might also involve higher side effects; hence, the importance of patient selection. For example, although the overall pCR rates were increased by adding lapatinib to trastuzumab (51.3% instead of 29.5%; P=0.0001), the frequencies of grade 3 diarrhoea, liver enzyme alterations, neutropenia and skin disorders were higher (Baselga et al, 2012). Better tolerance was reported when adding pertuzumab to trastuzumab (Gianni et al, 2012). When poor responders are to be selected for novel treatment strategies within clinical trials, the role of early metabolic response prediction is important.
Because data from the present prospective study were acquired in a single institution, the criteria for metabolic response that we raise need validation in large multicentric studies.
Conclusion
In patients with HER2+ breast cancer, interim 18F-FDG-PET/CT after two cycles of chemotherapy allowed early identification of poor metabolic responders who are at high risk of residual tumour after completion of NAT with chemotherapy+trastuzumab. Because alternative strategies are now available (e.g., chemotherapy+dual anti-HER2 blockade, addition of an antiangiogenic), early prediction can be helpful for patient selection within clinical trials.
Change history
03 September 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J, Jeannin JF, Coudert B (2006) Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 94: 259–267.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horváth Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M NeoALTTO Study Team (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379: 633–640.
Boellaard R, O'Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, Marsden PK, Tatsch K, Hoekstra CJ, Visser EP, Arends B, Verzijlbergen FJ, Zijlstra JM, Comans EF, Lammertsma AA, Paans AM, Willemsen AT, Beyer T, Bockisch A, Schaefer-Prokop C, Delbeke D, Baum RP, Chiti A, Krause BJ (2010) FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imag 37: 181–200.
Cochet A, Kerrou K, Nabholtz JM, Cachin F, Pierga JY, Champion L, Ferrero JM, Darcourt J, Petit T, Bourahla K, Bougnoux P, Baulieu JL, Dupre PF, Salaun PY, Bachelot TD, Mognetti T, Coeffic DE, Mesnard N, Coudert B, Berriolo-Riedinger A (2012) An open-label randomized, multicenter, phase II study on neoadjuvant treatment with trastuzumab plus docetaxel versus trastuzumab plus docetaxel plus bevacizumab according to positron emission tomography (PET) value modification in patients with early stage HER2-positive breast cancer (AVATAXHER): Design description. J Clin Oncol 30: (): (Suppl; abstr TPS646).
Fuster D, Duch J, Paredes P, Velasco M, Muñoz M, Santamaría G, Fontanillas M, Pons F (2008) Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol 26: 4746–4751.
Gebhart G, Flamen P, Robles Barba J, Holmes E, Garcia C, Cortes Romera M, Eidtmann H, Baselga J, Gamez Cenzano C (2012) FDG-PET/CT for early prediction of response to neoadjuvant anti-HER2 therapies in HER2-positive breast cancer (BC) patients. J Nucl Med 53: 60 (Suppl; Abstracts)..
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375: 377–384.
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13: 25–32.
Groheux D, Giacchetti S, Delord M, Hindié E, Vercellino L, Cuvier C, Toubert ME, Merlet P, Hennequin C, Espié M (2013a) 18F-FDG PET/CT in staging patients with locally advanced or inflammatory breast cancer: comparison to conventional staging. J Nucl Med 54: 5–11.
Groheux D, Giacchetti S, Espié M, Rubello D, Moretti JL, Hindié E (2011a) Early monitoring of response to neoadjuvant chemotherapy in breast cancer with (18)F-FDG PET/CT: defining a clinical aim. Eur J Nucl Med Mol Imag 38: 419–425.
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, de Roquancourt A, Hamy AS, Cuvier C, Vercellino L, Hindié E (2011b) Correlation of high (18)F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imag 38: 426–435.
Groheux D, Hatt M, Hindié E, Giacchetti S, De Cremoux P, Lehmann-Che J, Martineau A, Marty M, Cuvier C, Cheze-Le Rest C, de Roquancourt A, Visvikis D, Espié M (2013b) Estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast tumors: early prediction of chemosensitivity with (18) F-fluorodeoxyglucose positron emission tomography/computed tomography during neoadjuvant chemotherapy. Cancer 119: 1960–1968.
Groheux D, Hindié E, Delord M, Giacchetti S, Hamy AS, de Bazelaire C, de Roquancourt A, Vercellino L, Toubert ME, Merlet P, Espié M (2012a) Prognostic impact of 18FDG-PET-CT findings in clinical stage III and IIB breast cancer. J Natl Cancer Inst 104: 1879–1887.
Groheux D, Hindié E, Giacchetti S, Delord M, Hamy AS, de Roquancourt A, Vercellino L, Berenger N, Marty M, Espié M (2012b) Triple-negative breast cancer: early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J Nucl Med 53: 249–254.
Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, Ravaioli A, Cavanna L, Giardina G, Musolino A, Untch M, Orlando L, Artioli F, Boni C, Generali DG, Serra P, Bagnalasta M, Marini L, Piacentini F, D'Amico R, Conte P (2012) Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB Study. J Clin Oncol 30: 1989–1995.
Hatt M, Groheux D, Martineau A, Espié M, Hindié E, Giacchetti S, de Roquancourt A, Visvikis D, Cheze-Le Rest C (2013) Comparison between 18F-FDG PET image-derived indices for early prediction of response to neoadjuvant chemotherapy in breast cancer. J Nucl Med 54: 341–349.
Humbert O, Berriolo-Riedinger A, Riedinger JM, Coudert B, Arnould L, Cochet A, Loustalot C, Fumoleau P, Brunotte F (2012) Changes in 18F-FDG tumor metabolism after a first course of neoadjuvant chemotherapy in breast cancer: influence of tumor subtypes. Ann Oncol 23: 2572–2577.
Koolen BB, Pengel KE, Wesseling J, Vogel WV, Vrancken Peeters MJ, Vincent AD, Gilhuijs KG, Rodenhuis S, Rutgers EJ, Valdés Olmos RA (2013) FDG PET/CT during neoadjuvant chemotherapy may predict response in ER-positive/HER2-negative and triple negative, but not in HER2-positive breast cancer. Breast S0960-9776 (13): 00002–00007.
NCCN Clinical Practice Guidelines in Oncology (2013) Breast Cancer. Version 3 (2013). Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
Pierga JY, Petit T, Delozier T, Ferrero JM, Campone M, Gligorov J, Lerebours F, Roché H, Bachelot T, Charafe-Jauffret E, Pavlyuk M, Kraemer S, Bidard FC, Viens P (2012) Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol 13: 375–384.
Rousseau C, Devillers A, Campone M, Campion L, Ferrer L, Sagan C, Ricaud M, Bridji B, Kraeber-Bodéré F (2011) FDG PET evaluation of early axillary lymph node response to neoadjuvant chemotherapy in stage II and III breast cancer patients. Eur J Nucl Med Mol Imag 38: 1029–1036.
Schwarz-Dose J, Untch M, Tiling R, Sassen S, Mahner S, Kahlert S, Harbeck N, Lebeau A, Brenner W, Schwaiger M, Jaenicke F, Avril N (2009) Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol 27: 535–541.
Smith TA, Appleyard MV, Sharp S, Fleming IN, Murray K, Thompson AM (2013) Response to trastuzumab by HER2 expressing breast tumour xenografts is accompanied by decreased hexokinase II, glut1 and [(18)F]-FDG incorporation and changes in (31)P-NMR-detectable phosphomonoesters. Cancer Chemother Pharmacol 71: 473–480.
Straver ME, Aukema TS, Olmos RA, Rutgers EJ, Gilhuijs KG, Schot ME, Vogel WV, Peeters MJ (2010) Feasibility of FDG PET/CT to monitor the response of axillary lymph node metastases to neoadjuvant chemotherapy in breast cancer patients. Eur J Nucl Med Mol Imag 37: 1069–1076.
Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, Camara O, Müller V, du Bois A, Kühn T, Stickeler E, Harbeck N, Höss C, Kahlert S, Beck T, Fett W, Mehta KM, von Minckwitz G, Loibl S (2011) Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol 29: 3351–3357.
Von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S (2012) Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30: 1796–1804.
Wang Y, Zhang C, Liu J, Huang G (2012) Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat 131: 357–369.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology; College of American Pathologists (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25: 118–145.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Groheux, D., Giacchetti, S., Hatt, M. et al. HER2-overexpressing breast cancer: FDG uptake after two cycles of chemotherapy predicts the outcome of neoadjuvant treatment. Br J Cancer 109, 1157–1164 (2013). https://doi.org/10.1038/bjc.2013.469
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.469
Keywords
This article is cited by
-
Molecular imaging of HER2 expression in breast cancer patients using a novel peptide-based tracer 99mTc-HP-Ark2: a pilot study
Journal of Translational Medicine (2023)
-
Breast cancer: treatment response assessment with FDG-PET/CT in the neoadjuvant and in the metastatic setting
Clinical and Translational Imaging (2023)
-
Novel applications of molecular imaging to guide breast cancer therapy
Cancer Imaging (2022)
-
Positron emission tomography in breast cancer: 18F- FDG and other radiopharmaceuticals
European Journal of Hybrid Imaging (2018)
-
Additional value of 18F-FDG PET/CT response evaluation in axillary nodes during neoadjuvant therapy for triple-negative and HER2-positive breast cancer
Cancer Imaging (2017)