Abstract
Background:
Medulloblastoma is the most common malignant childhood brain tumour. Aberrant activation of the WNT/β-catenin pathway occurs in approximately 25% of medulloblastomas. However, its role in medulloblastoma pathogenesis is not understood.
Methods:
We have developed a model of WNT/β-catenin pathway-activated medulloblastoma. Pathway activation was induced in a Myc immortalised cerebellar progenitor cell line through stable expression of Wnt1. In vitro and in vivo analysis was undertaken to understand the effect of pathway activation and identify the potential cell of origin.
Results:
Tumours that histologically resembled classical medulloblastoma formed in vivo using cells overexpressing Wnt1, but not with the control cell line. Wnt1 overexpression inhibited neuronal differentiation in vitro, suggesting WNT/β-catenin pathway activation prevents cells terminally differentiating, maintaining them in a more ‘stem-like’ state. Analysis of cerebellar progenitor cell markers demonstrated the cell line resembled cells from the cerebellar ventricular zone.
Conclusion:
We have developed a cell line with the means of orthotopically modelling WNT/β-catenin pathway-activated medulloblastoma. We provide evidence of the role pathway activation is playing in tumour pathogenesis and suggest medulloblastomas can arise from cells other than granule cell progenitors. This cell line is a valuable resource to further understand the role of pathway activation in tumorigenesis and for investigation of targeted therapies.
Similar content being viewed by others
Main
The most common solid tumours during childhood are those arising in the central nervous system (CNS). Medulloblastomas occur in the cerebellum and are the most common malignant paediatric brain tumours (Ellison, 2002). Over recent years, treatments have improved with 5-year survival rates for patients with medulloblastoma of 60–70% (Louis et al, 2007). However, one third of patients still die and many surviving patients suffer long-term side effects from treatments (Gilbertson and Ellison, 2008).
Recent expression array studies have identified subgroups of medulloblastoma associated with different molecular pathways (Thompson et al, 2006; Kool et al, 2008; Northcott et al, 2011). One subgroup was characterised by aberrant activation of the WNT/β-catenin pathway. Additional studies have identified WNT/β-catenin pathway activation in 25% medulloblastomas with the majority associated with activating mutations in β-catenin (Ellison et al, 2005; Clifford et al, 2006; Gajjar et al, 2006; Rogers et al, 2009). Pathway activation has also been shown to be associated with a better clinical outcome (Ellison et al, 2005; Gajjar et al, 2006).
The WNT/β-catenin pathway plays a key role in many cellular functions related to tumorigenesis including cell proliferation, migration and differentiation. β-Catenin is the key downstream effecter. When the pathway is inactive, β-catenin is bound to a cytoplasmic complex containing adenomatous polyposis coli, axin1 and glycogen synthase kinase 3β. Glycogen synthase kinase 3β phosphorylates β-catenin which targets the protein for degradation through the ubiquitin-proteasome system (Morin, 1999). Upon pathway activation, the cytoplasmic complex is inhibited stabilising β-catenin and allowing it to translocate to the nucleus where it acts as a transcriptional cofactor of TCF and LEF and leads to the transcription of target genes including myc (MYC) and cyclin D1 (CCND1) (He et al, 1998; Tetsu and McCormick, 1999). In the majority of medulloblastoma cases, WNT/β-catenin pathway activation is caused by mutations in β-catenin that are thought to disrupt the phosphorylation sites that target the protein for degradation (Ellison et al, 2005; Clifford et al, 2006; Gajjar et al, 2006).
The WNT/β-catenin pathway plays a key role in CNS development including the cerebellum. Loss of Wnt1 or β-catenin in mouse models caused severe abnormalities in midbrain and cerebellar development by preventing specification of the midbrain–hindbrain boundary (McMahon and Bradley, 1990; Thomas and Capecchi, 1990; Schuller and Rowitch, 2007). The pathway also regulates stem cell proliferation (Chenn and Walsh, 2002; Israsena et al, 2004), and the balance between progenitor cell expansion and differentiation (Zechner et al, 2003).
The cell of origin of medulloblastoma has long been debated. Cerebellar neurons are generated from two progenitor cell zones, the cerebellar ventricular zone (VZ) and the rhombic lip (RL) (Millen and Gleeson, 2008). Granule neurons originate from the RL. The majority of other cerebellar neurons, as well as glial cells, originate from the VZ (Hatten and Roussel, 2011). Medulloblastomas were thought to arise from progenitors of granule neurons found in the external granule layer of the cerebellum (Louis et al, 2007). However, researchers have suggested that different subtypes of medulloblastoma arise from distinct progenitor cells, with WNT/β-catenin pathway subgroup tumours originating from progenitor cells in the VZ (Katsetos et al, 1995; Louis et al, 2007; Gilbertson and Ellison, 2008; Sutter et al, 2010). In a recent study by Gibson et al (2010), tumours were generated from dorsal brain stem progenitor cells in the lower RL in a mouse model of WNT/β-catenin pathway associated medulloblastoma.
The biological effect of WNT/β-catenin pathway activation in medulloblastoma is unknown. Pathway activation has been associated with a better prognosis, which could suggest activation is a disadvantage for the tumour. However, it seems unlikely a tumour would retain cells with an activating mutation unless it played some role in tumorigenesis. We hypothesised that pathway activation plays a fundamental role in tumorigenesis. To understand the role of WNT/β-catenin pathway activation in medulloblastoma, we stably expressed Wnt1, to activate the WNT/β-catenin pathway, in Myc immortalised cerebellar progenitor cells (Snyder et al, 1992). We show that WNT/β-catenin pathway activation enables tumours to form in vivo and propose that WNT/β-catenin pathway does this by inhibiting the ability of the cells to differentiate, thereby maintaining them in their proliferative state. We found expression of nerve growth factor receptor (NGFR) was downregulated in the Wnt1-expressing cell line. Signalling through NGFR, by nerve growth factor (NGF), can induce neuronal differentiation, suggesting downregulation of NGFR expression could be one mechanism by which WNT/B-catenin pathway activation inhibited differentiation. Analysis of cerebellar progenitor cell markers suggested the C17.2 line did not resemble granule cell progenitors, supporting evidence that medulloblastomas with WNT/β-catenin pathway activation do not originate from the external granule layer.
Materials and Methods
Stable cell line generation
The pCA-EGFP vector was used to generate a Wnt1 expression clone. EGFP was removed and Wnt1 cDNA was cloned into the vector. The C17.2 cell line generated from mouse cerebellar progenitor cells (Snyder et al, 1992) was stably transfected with the Wnt1 expression construct. Cells expressing Wnt1 were selected using 2 mg ml−1 of zeocin (Invitrogen, Carlsbad, CA, USA).
Cell culture conditions
C17.2 cells were cultured in standard humidified incubators at 5% CO2 in Dulbecco’s modified Eagle’s medium/L-glutamine (Invitrogen) supplemented with 10% fetal bovine serum (PAA Laboratories, Yeovil, UK), 5% horse serum (Invitrogen) and antibiotics. Under differentiation conditions, the media above was replaced with neurobasal medium (Invitrogen) containing B27 supplement (Invitrogen), 2 mM L-glutamine (Sigma-Aldrich, Poole, UK) and NGF (Millipore, Watford, UK) at a final concentration of 200 ng ml−1. For the Wnt1 stable cell line, media was supplemented with 0.5 mg ml−1 of zeocin (Invitrogen). For calculation of cell doubling times in low-serum conditions, 2% serum was used.
RT–PCR
Independent samples were taken from cell lines for analysis. RNA was extracted from snap-frozen cell pellets using the mirVana miRNA Isolation kit (Invitrogen), following the manufacturers’ instructions. After extraction, 1 μg of each RNA sample was treated with 2 U DNAse and incubated at 37 °C for 15 min followed by 10 min at 65 °C. cDNA synthesis was carried out using the Revertaid cDNA synthesis kit (Fermentas, St Leon-Rot, Germany). Briefly, 500 ng of RNA was incubated with 200 U reverse transcriptase and 500 ng of OligodT primer at 42 °C for 1 h followed by 70 °C for 5 min. Twenty units of RiboLock RNase inhibitor was also included in the reaction mix.
Primers used for PCR are displayed in Table 1. One microliter of cDNA was incubated with 1 × Biomix Red, and forward and reverse primers (1 nM) in a final volume of 10 μl. The PCR conditions were: 95 °C for 10 min followed by 40 cycles of 95 °C for 30 s, optimised primer annealing temperature for 1 min and 72 °C for 1 min. Products were visualised by electrophoresis through a 2% agarose gel at 120 V for 30 min. For primers using a touchdown programme the annealing temperature was decreased by 1 °C each cycle for the first 10 cycles, starting from 65 °C. GAPDH was used as a housekeeping gene in all experiments.
Quantitative PCR
Quantitative PCR reactions were carried out as previously described (Rogers et al, 2012). Primer sequences and optimised annealing temperatures are given in Table 1. Three independent cDNA samples from each cell line were used for each analysis. Data was normalised using Gapdh. Relative expression compared to one C17.2 cDNA sample was calculated using the Pfaffl equation (Pfaffl, 2001).
Immunofluorescence
Cells were fixed by incubating with 4% PFA for 20 min. Cells were washed 3 × 5 min in PBST (PBS, 0.1% Tween). Cells were permeabilised and blocked in 5% normal goat serum (NGS, Invitrogen) and 0.25% Triton X-100 in PBS at room temperature for 1 h. Cells were washed in PBST for 3 × 5 min. Primary antibodies were prepared in 2% NGS and 0.1% Triton X-100 in PBS. Cells were then incubated with the primary antibody at 4 °C overnight. The following antibodies were used; mouse anti-β-catenin (1 in 200, 2677 Cell Signaling Technology, Danvers, MA, USA) and rabbit anti-TUJ1 (1 in 500, Covance, Harrogate, UK). Cells were washed 3 × 5 min in PBST then incubated with the appropriate secondary antibody; Alexa Fluor 555 goat anti-mouse (1 in 500, Invitrogen) or Alexa Fluor 488 goat anti-rabbit antibody (1 in 500, Invitrogen) at room temperature for 1 h in the dark. After three further washes in PBST, cells were mounted using Vectashield containing 4′,6-diamidinophenylindole (Vector Laboratories, Peterborough, UK). Images were obtained using a Leica DMRM fluorescent microscope (Nikon, Digital sight-USB (H), Kingston upon Thames, UK) equipped with a Nikon digital camera. NIS elements software was used to capture images (Nikon).
Western blot
Western blotting was carried out as previously described (Levesley et al, 2011). Membranes were incubated with primary antibodies to Wnt1 (1 in 500, Abcam, Cambridge, UK), Ngfr (1 in 1000, Cell Signaling Technology) and Gapdh (1 in 100, Abcam) for 1 h at room temperature.
Intracranial injection of cells into rats
Adult male Sprague-Dawley rats were housed with libitum access to food and water at a 12-h light/dark cycle. Animals were treated according to the guidelines of the European Community and local ethics committee. The animals were immune suppressed using cyclosporine A (10 mg kg−1 per day). Cells at 85–90% confluence were prepared for transplantation and stored on wet ice. Animals were anesthetised with isofluorane (1–2.5% in an oxygen/70% nitrous oxide mixture) and stereotaxically injected with 1 μl of cell suspension (200 000 cells μl−1) at two sites directly overlying the substantia nigra. Cells were injected slowly through a 22-guage, 10 μl Hamilton syringe, and the needle was left in place for 2 min after completion of injection. For each cell line, four animals were transplanted. After 8 weeks, brains were excised. Frozen sections were cut and stained with haematoxylin and eosin for subsequent review by a pathologist.
Immunohistochemistry
The following antibodies were used; rabbit anti-Ki-67 (SP6) (1 in 200, Neomarkers, Fremont, CA, USA), rabbit anti-β-catenin (1 in 250, 9582 Cell Signaling Technology) and rabbit anti-Myc (1 in 100, Abcam). Antigen retrieval was performed by incubating sections in sodium citrate buffer (pH 6) in a steamer for 40 min. Endogenous protein was blocked by incubating with 5% NGS for 10 min. Sections were then incubated with a peroxidase block (Dako, Ely, UK) for 5 min. Ki-67 and β-catenin antibodies were incubated at 4 °C overnight. Myc antibody was incubated for 1 h at room temperature. Detection was undertaken using the EnVision detection system peroxidase/DAB (Dako). Stained sections were reviewed by a pathologist.
Electron microscopy
Frozen sections were fixed in 2.5% glutaraldehyde in cacodylate buffer for 24 h followed by 1% osmium tetroxide for 1 h. This was followed by standard EM processing using TAAB TER resin, then inverting a resin-filled capsule over the section on the slide before polymerisation at 65 °C overnight. The subsequent resin block containing the section was removed from the glass slide and trimmed. A 0.5-μm section was cut using a Leica UM7 ultramicrotome before staining with toluidine blue in 1% borax (Leica Microsystems, Milton Keynes, UK). Slides were examined using a light microscope and an area selected for electron microscopy. Eighty nanometer sections were cut from the selected area and mounted on a copper grid and stained with uranyl acetate in ethanol followed by lead citrate. The stained sections were examined in a JEOL 1010 transmission electron microscope at 100 KV and appropriate images recorded using an integrated digital camera system (Olympus-SIS, Munster, Germany).
Results
Construction of a cerebellar progenitor cell line with stable WNT/β-catenin pathway activation
We used a cerebellar progenitor cell line (C17.2) immortalised by stable expression of Myc (Snyder et al, 1992) to investigate the role of the WNT/β-catenin pathway activation in medulloblastoma. Pathway activation was induced by constructing a cell line with stable expression of Wnt1. Wnt1 cDNA was cloned into the pCA-EGFP vector and then transfected into the C17.2 cells. Multiple clones of cells were isolated. The stable Wnt1-expressing cell line (C17.2-Wnt1) selected for further analysis did not show abnormal morphology or cell growth rate compared with the other Wnt1 lines. Stable Wnt1 expression was confirmed by RT–PCR and western blot (Figures 1A and B). No Wnt1 gene or protein expression was seen in the original C17.2 line. Myc gene expression was retained in the C17.2-Wnt1 cell line with no significant difference in expression level compared with C17.2 (Figure 1C). The morphology of C17.2-Wnt1 was slightly altered relative to C17.2. The C17.2 cell line changed in appearance during culture, sometimes looking epithelial (Figure 1D) and sometimes displaying cell processes (Figure 1E). The C17.2-Wnt1 line looked uniformly epithelial with a more rounded cell shape compared with C17.2 (Figure 1F). To demonstrate Wnt1 expression activated the WNT/β-catenin pathway, we initially looked at the expression of the pathway target Axin2 by RT–PCR. Expression was only seen in the C17.2-Wnt1 line (Figure 1A). We also analysed the intra-cellular location of β-catenin. Upon pathway activation, β-catenin is stabilised and translocates to the nucleus to induce transcription of target genes. Nuclear localisation of β-catenin was only seen in C17.2-Wnt1 (Figure 1G).
Characterisation of C17.2 and C17.2-Wnt1 cell lines. Wnt1 gene and protein expression was confirmed in C17.2-Wnt1 by RT–PCR and western blot, respectively (A and B). WNT/β-catenin pathway activation in C17.2-Wnt1 was confirmed by Axin2 expression. Expression of the WNT/β-catenin pathway target Axin2 was only seen in the C17.2-Wnt1 line (A). The housekeeping gene Gapdh demonstrated similar gene and protein expression levels in both C17.2 and C17.2-Wnt1 (A and B). Three repeats generated from independent samples are displayed for each cell line; C1, C2 and C3 from C17.2 and W1, W2, W3 from C17.2-Wnt1. Myc gene expression was not altered after Wnt1 expression in the C17.2 cell line as measured by qPCR (C). Stable overexpression of Wnt1 altered the morphology of the cells. The appearance of C17.2 was sometimes epithelial (D) and sometimes displayed cell processes (E). C17.2-Wnt1 was uniformly epithelial with a more rounded cell shape compared with C17.2 (F). The cellular location of β-catenin was investigated using immunofluorescence. In C17.2, only membrane and cytoplasmic staining of β-catenin was seen. In C17.2-Wnt1, nuclear staining, indicating pathway activation, was also seen (G). Abbreviation: Neg=negative control.
Functional effect of WNT/β-catenin pathway activation
We investigated the functional effect of WNT/β-catenin pathway activation in vitro to try to gain an understanding of what role pathway activation is playing in tumorigenesis. We investigated cell proliferation by calculating doubling times in low- and high-serum conditions. However, we found no significant difference between the two cell lines (data not shown). We also investigated cell migration using a scratch assay but again saw no difference in the presence or absence of WNT/β-catenin pathway activation (data not shown).
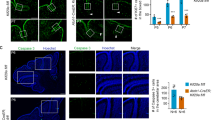
We looked at the cells’ ability to undergo neuronal differentiation by culturing the cells in differentiation media, containing NGF. After 1 day, C17.2 cells differentiated into neurons. However, even after 7 days, the C17.2-Wnt1 cell line had not differentiated (Figures 2A–D). We investigated this further by staining fluorescently for the neuronal marker beta-tubulin (Tuj1). C17.2 cells grown in normal or differentiation culture media displayed expression of Tuj1, with the differentiated cells displaying a higher intensity signal. The C17.2-Wnt1 cells displayed no staining under either condition (Figure 2E).
WNT/β-catenin pathway activation inhibits neuronal differentiation. Cells were grown in normal growth media (GM) or differentiation media (DM). After 1 day in DM, C17.2 cells lost the epithelial morphology seen when in GM (A) and gained a neuronal appearance (B). C17.2-Wnt1 retained the same appearance in GM (C) or DM (D) with no evidence of neuronal differentiation. C17.2 cells displayed some TUJ1 staining in GM with an increased intensity of staining when the cells underwent neuronal differentiation in DM. C17.2-Wnt1 cells displayed no TUJ1 staining under growth or differentiation conditions (E). Nerve growth factor receptor (Ngfr) gene expression was analysed in three independent samples from C17.2 and C17.2-Wnt1 by RT–PCR. Nerve growth factor receptor expression was seen in C17.2 (C1–C3). No expression or a very low level of expression was seen in C17.2-Wnt1 (W1-W3) (F). Expression of the housekeeping gene Gapdh was seen in all samples. Nerve growth factor receptor protein expression, measured by western blot, was seen in C17.2 (C1, C2) but lost in C17.2-Wnt1 (W1, W2) (G). Protein expression of Gapdh was seen in all samples. Abbreviation: Neg=negative control.
To investigate further the role the WNT/β-catenin pathway is having in the decision to undergo differentiation, we investigated the expression of a number of genes involved in neuronal differentiation. To stimulate differentiation in culture, we used NGF. We therefore looked at the expression of the two receptors of this protein; Ngfr and neurotrophic tyrosine kinase receptor type 1 (Ntrk1). Gene expression of Ntrk1 was not seen in either cell line (data not shown). Nerve growth factor receptor (Ngfr) gene expression was seen in C17.2; however, expression was decreased in C17.2-Wnt1 (Figure 2F). Nerve growth factor receptor (Ngfr) protein expression was seen in C17.2 but was lost in C17.2-Wnt1 (Figure 2G).
Cells with WNT/β-catenin pathway activation form tumours in vivo
C17.2 and C17.2-Wnt1 cells were injected orthotopically into immunosupressed rats. As previously published (Snyder et al, 1992), C17.2 cells did not form tumours. However, with induced WNT/β-catenin pathway activation (C17.2-Wnt1 cells), tumours rapidly formed after 4 weeks. All four rats transplanted with C17.2-Wnt1 cells developed tumours. Histologically, tumours resembled primitive neuroectodermal tumours (PNETs), consistent with the appearance of classical medulloblastoma. Tumours were well circumscribed with no infiltration into the surrounding brain tissue (Figures 3A and B). Ki67 staining revealed a high proliferation rate similar to that seen in medulloblastoma (Figure 3C). β-Catenin staining revealed nuclear localisation of the protein, suggesting the WNT/β-catenin pathway was active in vivo, as well as in vitro (Figure 3D). Tumours also displayed Myc protein expression (Figure 3E). Electron microscopy was used to look for features of differentiation. Very few features of neuronal differentiation such as neurosecretory vesicles were seen, consistent with a PNET (Figures 3F and G). Our results demonstrated that cerebellar progenitor cells with stable Myc expression and WNT/β-catenin pathway activation became tumorigenic.
C17.2-Wnt1 cells formed tumours in vivo. Hematoxylin and eosin staining shows a tumour similar in appearance to a PNET. Tumours were well circumscribed with no infiltration into the surrounding brain tissue (A and B). Ki67 staining revealed a proliferation rate in the tumours similar to those seen in medulloblastoma (C). Nuclear localisation of β-catenin was seen in the tumours, indicating WNT/β-catenin pathway activation (D). Tumours also displayed myc protein expression (E). Electron microscopy revealed very few features of differentiation such as neurosecretory vesicles (red arrow) (F and G). Features were consistent with a PNET.
C17.2 cells express stem cell markers of the cerebellar VZ
To investigate the cell of origin of medulloblastomas with WNT/β-catenin pathway activation, we looked at what cell type C17.2 cells resemble. We analysed the expression levels of genes that have been linked to different cerebellar progenitor cell types using RNA extracted from cells grown in normal or differentiation media using RT–PCR. For granule cell progenitors, we investigated Atoh1 and Cntn2 (Ben-Arie et al, 1997; Backer et al, 2002; Bizzoca et al, 2003), whereas ptf1a, Meis1 and Lhx2 were used as markers of cells in the cerebellar VZ (Hoshino et al, 2005; Morales and Hatten, 2006; Millen and Gleeson, 2008). We also investigated the neural stem cell marker Sox2 and the post mitotic marker of Purkinje cells Calb1 (Wassef et al, 1985).
Expression was only seen for Ptf1a, Meis1 and Sox2, suggesting the C17.2 cells are more like stem cells found in the VZ than granule cell progenitors in the RL. Ptf1a expression was seen in a subset of samples from C17.2 but not in C17.2-Wnt1 (Figure 4A). During culture, the appearance of C17.2 varied sometimes appearing more differentiated and sometimes more epithelial. This variation may have influenced when Ptf1a expression was seen. Meis1 and Sox2 expression was seen in all samples analysed from both cell lines. Quantitative PCR revealed that Meis1 displayed a similar level of gene expression in both cell lines (Figure 4B). Sox2 gene expression was four-fold higher in C17.2 compared with C17.2-Wnt1 (Figure 4B). No expression was seen for Atoh1 or Cntn1, suggesting, in this model, the cells generating tumours did not resemble granule cell progenitors.
Gene expression of cerebellar progenitor markers. Expression was analysed in samples extracted from cells grown in normal growth media (C17.2; C1-4, C17.2-Wnt1; W1-4) and cells grown in differentiation media (C17.2; Cd1-2, C17.2-Wnt1; Wd1-2). Ptf1a expression was seen in a subset of C17.2 cells measured by RT–PCR (A). Expression of the housekeeping gene gapdh was seen in all samples. Meis1 and Sox2 expression was seen in all samples analysed from both cell lines. Quantitative PCR revealed equal expression of Meis1 in C17.2 and C17.2-Wnt1 cell lines. Sox2 displayed four-fold higher expression in C17.2 compared with C17.2-Wnt1 (B). Abbreviation: Neg=negative control.
Discussion
We have generated a cell line with the means to orthotopically model medulloblastoma with WNT/β-catenin pathway activation. Previous studies have demonstrated that tumours with WNT/β-catenin pathway activation account for 25% of medulloblastomas. However, the role pathway activation is playing in the development of these tumours is not understood. Therefore, it is important to generate models that can be used to study the pathology of this disease and as a resource for developing and testing new treatments. We provide evidence of the role the WNT/β-catenin pathway may be having in medulloblastoma. Our data suggest pathway activation is contributing to tumorigenesis by inhibiting neuronal differentiation of progenitor cells therefore maintaining them in a proliferative and more ‘stem-like’ state.
Aberrant activation of the WNT/β-catenin pathway in a subset of medulloblastomas has been demonstrated in a number of studies (Ellison et al, 2005; Clifford et al, 2006; Thompson et al, 2006; Rogers et al, 2009). However, the biological effect of pathway activation and how this influences tumorigenesis is still unknown. Pathway activation in medulloblastoma has also been shown to be associated with a better prognosis, suggesting it is a disadvantage to the tumour (Ellison et al, 2005; Gajjar et al, 2006). However, we hypothesised that the pathway is involved in the development of medulloblastoma and that therefore the reason for the association with a better prognosis is that this subgroup is less aggressive or more susceptible to current treatments than other medulloblastomas with different molecular aberrations.
We demonstrated that WNT/β-catenin pathway activation in cerebellar progenitor cells caused the cells to form tumours in vivo that histologically resembled classical medulloblastoma, confirming our hypothesis that pathway activation is involved in tumorigenesis. In our model, pathway activation may be working in conjunction with Myc, as the gene is stably expressed in the C17.2 cell line and protein expression was retained in tumours generated in vivo. Previously reported data has shown that MYC expression is seen in medulloblastomas with WNT/β-catenin pathway activation (Kool et al, 2008; Northcott et al, 2011), suggesting our model is representative of this clinical subgroup. Analysis of previously published medulloblastoma expression array data ((GSE10237) (Kool et al, 2008)) demonstrated that tumours with WNT/β-catenin pathway activation displayed a significantly higher level of Myc expression compared with the rest of the tumours (P=0.006), suggesting the two may cooperate in patient tumours (Supplementary Figure 1). In a mouse model of CNS PNET, β-catenin mutations alone did not induce tumour formation. However, in combination with Myc expression and p53 knockout tumours developed (Momota et al, 2008).
In our model, WNT/β-catenin pathway activation inhibited neuronal differentiation in vitro. The WNT/β-catenin pathway plays a complex role in CNS development. WNT/β-catenin pathway signals are involved in the positioning of the cerebellum along the anterior–posterior axis of the neural tube (Sato et al, 2004). The pathway has also been shown to influence the decision of neuronal progenitor cells to proliferate or differentiate. At different developmental stages, pathway activation has either been shown to stimulate proliferation or differentiation (Chenn and Walsh, 2002; Viti et al, 2003; Zechner et al, 2003; Israsena et al, 2004). In the majority of studies, pathway activation was found to stimulate progenitor cell proliferation at early development stages and neuronal differentiation during later stages (Hirsch et al, 2007). Less is known about how the WNT/β-catenin pathway specifically affects progenitor cells in the cerebellum. However, a recent study demonstrated that WNT/β-catenin pathway activation promoted proliferation and impaired differentiation of neural stem cells in the developing cerebellum (Pei et al, 2012).
Our data suggest the C17.2 cell line is derived from progenitor cells at a stage in development when they are prevented from differentiating in response to WNT/β-catenin pathway signals. These findings suggest that activation of WNT/β-catenin pathway in our model may be causing tumour formation by preventing differentiation and maintaining the cells in a more primitive, proliferating state. We saw no difference in the rate of proliferation between C17.2 and C17.2-Wnt1 cells. This may be due to the fact that the C17.2 cell line was originally immortalised using Myc. We suggest that myc stimulates proliferation of the cells and WNT/β-catenin pathway activation prevents the cells from responding to signals to differentiate. Similarly, in a mouse model of CNS PNET, induced by WNT/β-catenin pathway activation in conjunction with Myc expression and p53 knockout, tumours displayed regions of differentiation, but not in cells displaying nuclear β-catenin, indicating pathway activation inversely correlated with differentiation (Momota et al, 2008).
Analysis of medulloblastoma expression array data revealed that genes displaying differential expression between the WNT/β-catenin pathway subgroup and other medulloblastomas included a number of genes linked to neuronal differentiation. This included upregulation of the fetal growth factor FGF20 and the epidermal growth factor receptor ERBB2, and downregulation of the neuronal marker MAP2 in the WNT subgroup (Kool et al, 2008; Northcott et al, 2011). Additionally, a recent study has shown increased expression of neuronal markers in medulloblastoma xenografts treated with drugs that can inhibit the WNT/β-catenin pathway (Cimmino et al, 2012).
We saw a downregulation of Ngfr expression in C17.2-Wnt1 cells compared with C17.2. Nerve growth factor can bind to two different cell receptors; NGFR and the tyrosine kinase NTRK1. Signalling through NGFR has been shown to stimulate many different responses including survival, differentiation and apoptosis (Friedman, 2000; Lee et al, 2001; Dechant and Barde, 2002; Lu et al, 2005). Nerve growth factor receptor is highly expressed on neurons and glia during CNS development (Cragnolini and Friedman, 2008). Neonatal NGFR-positive neural stem cells have been shown to have neurogenic potential that was enhanced by treatment with neurotrophins including NGF. However, this enhancement was not seen using stem cells from NGFR−/− mice (Young et al, 2007). Nerve growth factor receptor is expressed in the developing cerebellum; however, its’ role is not fully understood (Barnes et al, 2009).
Nerve growth factor receptor expression has also been seen in medulloblastoma. However, expression was not seen in medulloblastomas with WNT/β-catenin pathway activation (Kool et al, 2008; Barnes et al, 2009). Analysis of previously published mRNA array expression data ((GSE10237) (Kool et al, 2008)) demonstrated WNT subgroup tumours displayed a low level of NGFR expression compared with the rest of the tumour cohort (Supplementary Figure 2). Nerve growth factor receptor expression has also shown higher expression in desmoplastic compared with classic medulloblastoma (Buhren et al, 2000; Kuchler et al, 2011). Consistent with these histological findings, WNT/β-catenin pathway activation has more frequently been seen in classic medulloblastomas and sonic hedgehog (Shh) pathway activation in desmoplasic tumours (Thompson et al, 2006).
No expression of the second receptor to NGF, Ntrk1, was seen in C17.2 or C17.2-Wnt1. Therefore, a loss of Ngfr expression in C17.2-Wnt1 means they lack any receptor to respond to NGF signals, which may explain the lack of neuronal differentiation seen in response to NGF treatment. As far as we are aware there is no evidence in the literature linking the WNT/β-catenin pathway to NGFR expression. Further investigation is needed to determine if the downregulation of expression seen in the C17.2-Wnt1 cells is a direct consequence of WNT/β-catenin signalling.
There has been much debate about the cell of origin of medulloblastoma. In the cerebellum, there are two distinct zones of progenitor cells; the VZ and the RL. The main type of cells generated from the RL are granule neurons with the majority of other cell types arising from the VZ (Hatten and Roussel, 2011). Medulloblastomas have been suggested to arise from granule cell precursors. However, genomic and expression data from medulloblastomas suggested that not all subtypes arise from these cells (Gilbertson and Ellison, 2008; Sutter et al, 2010). A more current interpretation proposes that medulloblastomas with WNT/β-catenin pathway activation may arise from progenitor cells in the VZ. In support of this hypothesis, aberrant activation of the WNT/β-catenin pathway induced proliferation and inhibited differentiation of neural stem cells from the cerebellar VZ but not granule progenitor cells (Pei et al, 2012). In a separate study, selected depletion of adenomatous polyposis coli in ATOH1-expressing cells lead to a decrease in cell proliferation and premature differentiation of cerebellar granule cells (Lorenz et al, 2011).
The lack of expression of granule cell markers Atoh1 and Cntn2 in both the C17.2 and C17.2-Wnt1 lines suggests the C17.2 cells did not resemble this lineage. The bHLH factor Ptf1a is essential for the generation of GABAergic neurons from the cerebellar VZ (Hoshino et al, 2005). C17.2 cells demonstrated expression of Ptf1a, suggesting the cells resembled VZ cells more than granule cell progenitors. This was supported by the expression of Meis1, which has been shown to be expressed by cells in the VZ (Morales and Hatten, 2006). The original C17.2 cell line was developed from cerebella of newborn mice (P4) (Ryder et al, 1990). At this stage of development, dividing granule cell progenitors are present in the RL. Neural stem cells, which originated in the VZ, are also present in the white matter (Lee et al, 2005). Therefore, the C17.2 line could have been generated from the neural stem cells rather than granule progenitor cells. However, it cannot be discounted that the immortalisation of the C17.2 cells with Myc may have affected the phenotype of the original cells the line was derived from, as Myc also plays a role in normal neuronal development (Hatton et al, 2006; Wey et al, 2010). It has also previously been demonstrated that immortalisation of granule cell precursors with SV40 T antigen caused the cells to lose their restricted fate and gain an ability to differentiate into multiple cell types (Gao and Hatten, 1994). Hence, it cannot be discounted that the original cells the C17.2 line was generated from could have been granule progenitor cells that were altered by Myc immortalisation.
The gene expression of cerebellar markers seen in C17.2 and C17.2-Wnt1 cells reflects what is seen in WNT subgroup medulloblastomas ((GSE10237) (Kool et al, 2008)). High Atoh1 and Cntn2 expression was seen in Shh sub-group tumours, but not in the WNT/β-catenin group. Meis1 expression was seen in both WNT and Shh groups. Sox2 displayed higher expression in the Shh group (Supplementary Figure 3). Ptf1a data was not available.
In the study by Gibson et al (2010), tumours resembling medulloblastoma were generated in mice with mutated β-catenin and p53 knockout. In this model, no tumours developed from cerebellar progenitors but instead arose from brain stem progenitor cells found in the lower RL. This region lines the ventral region of the fourth ventricle, with the cerebellum located on the dorsal side. They also found that the normal migration and differentiation of these cells was impaired in the tumour model, agreeing with the inhibition of neuronal differentiation we saw in our study. In the model developed by Gibson et al, tumours developed from a combination of WNT/β-catenin pathway activation and TP53 knockout in contrast to our model, where Myc expression was used which may explain why they did not see tumours develop from cerebellar progenitor cells. In addition, considering the complex role the WNT/β-catenin pathway has in CNS development, the timing of induced pathway activation may be important in determining where tumours arise. In a recent study, a transient increase in proliferation and disruption of normal cerebellar development occurred in neural stem cells from the cerebellar VZ when the WNT/β-catenin pathway was aberrantly activated, suggesting at least some WNT subgroup tumours may develop from cerebellar progenitors (Pei et al, 2012).
In conclusion, we have developed a cell line that provides the means to orthotopically model WNT/β-catenin pathway-activated medulloblastoma. We suggest that inhibition of progenitor cell differentiation, therefore maintaining them in a proliferative and more ‘stem-like’ state, is the mechanism by which pathway activation is involved in tumorigenesis, which in turn may be achieved through downregulated expression of NGFR. This evidence suggests pathway activation is playing a key role in tumour development and is therefore an important treatment target for this subset of medulloblastomas. Our data also demonstrates that progenitor cells expressing stem cell markers of the cerebellar VZ have the potential to develop tumours that resemble medulloblastoma. As tumours with WNT/β-catenin pathway activation account for 25% of medulloblastomas, understanding how the pathway is involved in tumorigenesis is important. This model can be used as a resource for future analysis of the role the pathway is playing in tumorigenesis and also for the development and testing of targeted therapies.
References
Backer S, Sakurai T, Grumet M, Sotelo C, Bloch-Gallego E (2002) Nr-CAM and TAG-1 are expressed in distinct populations of developing precerebellar and cerebellar neurons. Neuroscience 113: 743–748
Barnes M, Eberhart CG, Collins R, Tihan T (2009) Expression of p75NTR in fetal brain and medulloblastomas: evidence of a precursor cell marker and its persistence in neoplasia. J Neurooncol 92: 193–201
Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY (1997) Math1 is essential for genesis of cerebellar granule neurons. Nature 390: 169–172
Bizzoca A, Virgintino D, Lorusso L, Buttiglione M, Yoshida L, Polizzi A, Tattoli M, Cagiano R, Rossi F, Kozlov S, Furley A, Gennarini G (2003) Transgenic mice expressing F3/contactin from the TAG-1 promoter exhibit developmentally regulated changes in the differentiation of cerebellar neurons. Development 130: 29–43
Buhren J, Christoph AH, Buslei R, Albrecht S, Wiestler OD, Pietsch T (2000) Expression of the neurotrophin receptor p75NTR in medulloblastomas is correlated with distinct histological and clinical features: evidence for a medulloblastoma subtype derived from the external granule cell layer. J Neuropathol Exp Neurol 59: 229–240
Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297: 365–369
Cimmino F, Scoppettuolo MN, Carotenuto M, De Antonellis P, Dato VD, De Vita G, Zollo M (2012) Norcantharidin impairs medulloblastoma growth by inhibition of Wnt/beta-catenin signaling. J Neurooncol 106: 59–70
Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, Ellison DW (2006) Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle 5: 2666–2670
Cragnolini AB, Friedman WJ (2008) The function of p75NTR in glia. Trends Neurosci 31: 99–104
Dechant G, Barde YA (2002) The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci 5: 1131–1136
Ellison D (2002) Classifying the medulloblastoma: insights from morphology and molecular genetics. Neuropathol Appl Neurobiol 28: 257–282
Ellison DW, Onilude OE, Lindsey JC, Lusher ME, Weston CL, Taylor RE, Pearson AD, Clifford SC (2005) Beta-catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J Clin Oncol 23: 7951–7957
Friedman WJ (2000) Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci 20: 6340–6346
Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ (2006) Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 7: 813–820
Gao WQ, Hatten ME (1994) Immortalizing oncogenes subvert the establishment of granule cell identity in developing cerebellum. Development 120: 1059–1070
Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, Finkelstein D, Pounds S, Weiss A, Patay Z, Scoggins M, Ogg R, Pei Y, Yang ZJ, Brun S, Lee Y, Zindy F, Lindsey JC, Taketo MM, Boop FA, Sanford RA, Gajjar A, Clifford SC, Roussel MF, McKinnon PJ, Gutmann DH, Ellison DW, Wechsler-Reya R, Gilbertson RJ (2010) Subtypes of medulloblastoma have distinct developmental origins. Nature 468: 1095–1099
Gilbertson RJ, Ellison DW (2008) The origins of medulloblastoma subtypes. Annu Rev Pathol 3: 341–365
Hatten ME, Roussel MF (2011) Development and cancer of the cerebellum. Trends Neurosci 34: 134–142
Hatton BA, Knoepfler PS, Kenney AM, Rowitch DH, de Alboran IM, Olson JM, Eisenman RN (2006) N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res 66: 8655–8661
He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW (1998) Identification of c-MYC as a target of the APC pathway. Science 281: 1509–1512
Hirsch C, Campano LM, Wohrle S, Hecht A (2007) Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp Cell Res 313: 572–587
Hoshino M, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura YV, Fukuda A, Fuse T, Matsuo N, Sone M, Watanabe M, Bito H, Terashima T, Wright CV, Kawaguchi Y, Nakao K, Nabeshima Y (2005) Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron 47: 201–213
Israsena N, Hu M, Fu W, Kan L, Kessler JA (2004) The presence of FGF2 signaling determines whether beta-catenin exerts effects on proliferation or neuronal differentiation of neural stem cells. Dev Biol 268: 220–231
Katsetos CD, Herman MM, Krishna L, Vender JR, Vinores SA, Agamanolis DP, Schiffer D, Burger PC, Urich H (1995) Calbindin-D28k in subsets of medulloblastomas and in the human medulloblastoma cell line D283 Med. Arch Pathol Lab Med 119: 734–743
Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, Troost D, Meeteren NS, Caron HN, Cloos J, Mrsic A, Ylstra B, Grajkowska W, Hartmann W, Pietsch T, Ellison D, Clifford SC, Versteeg R (2008) Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One 3: e3088
Kuchler J, Hartmann W, Waha A, Koch A, Endl E, Wurst P, Kindler D, Mikeska T, Goodyer CG, Buttner R, Schilling K, Pietsch T (2011) p75(NTR) induces apoptosis in medulloblastoma cells. Int J Cancer 128: 1804–1812
Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ (2005) Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci 8: 723–729
Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294: 1945–1948
Levesley J, Lusher ME, Lindsey JC, Clifford SC, Grundy R, Coyle B (2011) RASSF1A and the BH3-only mimetic ABT-737 promote apoptosis in pediatric medulloblastoma cell lines. Neuro Oncol 13: 1265–1276
Lorenz A, Deutschmann M, Ahlfeld J, Prix C, Koch A, Smits R, Fodde R, Kretzschmar HA, Schuller U (2011) Severe alterations of cerebellar cortical development after constitutive activation of Wnt signaling in granule neuron precursors. Mol Cell Biol 31: 3326–3338
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) 114: 97–109
Lu B, Pang PT, Woo NH (2005) The yin and yang of neurotrophin action. Nat Rev Neurosci 6: 603–614
McMahon AP, Bradley A (1990) The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell 62: 1073–1085
Millen KJ, Gleeson JG (2008) Cerebellar development and disease. Curr Opin Neurobiol 18: 12–19
Momota H, Shih AH, Edgar MA, Holland EC (2008) c-Myc and beta-catenin cooperate with loss of p53 to generate multiple members of the primitive neuroectodermal tumor family in mice. Oncogene 27: 4392–4401
Morales D, Hatten ME (2006) Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J Neurosci 26: 12226–12236
Morin PJ (1999) beta-catenin signaling and cancer. Bioessays 21: 1021–1030
Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD (2011) Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29: 1408–1414
Pei Y, Brun SN, Markant SL, Lento W, Gibson P, Taketo MM, Giovannini M, Gilbertson RJ, Wechsler-Reya RJ (2012) WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development 139: 1724–1733
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45
Rogers HA, Kilday JP, Mayne C, Ward J, Adamowicz-Brice M, Schwalbe EC, Clifford SC, Coyle B, Grundy RG (2012) Supratentorial and spinal pediatric ependymomas display a hypermethylated phenotype which includes the loss of tumor suppressor genes involved in the control of cell growth and death. Acta Neuropathol 123: 711–725
Rogers HA, Miller S, Lowe J, Brundler MA, Coyle B, Grundy RG (2009) An investigation of WNT pathway activation and association with survival in central nervous system primitive neuroectodermal tumours (CNS PNET). Br J Cancer 100: 1292–1302
Ryder EF, Snyder EY, Cepko CL (1990) Establishment and characterization of multipotent neural cell lines using retrovirus vector-mediated oncogene transfer. J Neurobiol 21: 356–375
Sato T, Joyner AL, Nakamura H (2004) How does Fgf signaling from the isthmic organizer induce midbrain and cerebellum development? Dev Growth Differ 46: 487–494
Schuller U, Rowitch DH (2007) Beta-catenin function is required for cerebellar morphogenesis. Brain Res 1140: 161–169
Snyder EY, Deitcher DL, Walsh C, Arnold-Aldea S, Hartwieg EA, Cepko CL (1992) Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68: 33–51
Sutter R, Shakhova O, Bhagat H, Behesti H, Sutter C, Penkar S, Santuccione A, Bernays R, Heppner FL, Schuller U, Grotzer M, Moch H, Schraml P, Marino S (2010) Cerebellar stem cells act as medulloblastoma-initiating cells in a mouse model and a neural stem cell signature characterizes a subset of human medulloblastomas. Oncogene 29: 1845–1856
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426
Thomas KR, Capecchi MR (1990) Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 346: 847–850
Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, Chintagumpala M, Adesina A, Ashley DM, Kellie SJ, Taylor MD, Curran T, Gajjar A, Gilbertson RJ (2006) Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 24: 1924–1931
Viti J, Gulacsi A, Lillien L (2003) Wnt regulation of progenitor maturation in the cortex depends on Shh or fibroblast growth factor 2. J Neurosci 23: 5919–5927
Wassef M, Zanetta JP, Brehier A, Sotelo C (1985) Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev Biol 111: 129–137
Wey A, Cerdeno VM, Pleasure D, Knoepfler PS (2010) c- and N-myc regulate neural precursor cell fate, cell cycle, and metabolism to direct cerebellar development. Cerebellum 9: 537–547
Young KM, Merson TD, Sotthibundhu A, Coulson EJ, Bartlett PF (2007) p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci 27: 5146–5155
Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, Birchmeier W, Birchmeier C (2003) Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol 258: 406–418
Acknowledgements
Funding was provided by the Joseph Foote Foundation and the Connie and Albert Taylor Charitable Trust. We would like to thank Dr Cerys Mayne, Dr Jennifer Ward and Dr Lisa Storer for their technical support; Trevor Gray for EM images; and Professor James Lowe for review of histological slides.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Rogers, H., Sousa, S., Salto, C. et al. WNT/β-catenin pathway activation in Myc immortalised cerebellar progenitor cells inhibits neuronal differentiation and generates tumours resembling medulloblastoma. Br J Cancer 107, 1144–1152 (2012). https://doi.org/10.1038/bjc.2012.377
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.377
Keywords
This article is cited by
-
The role of the WNT/β-catenin pathway in central nervous system primitive neuroectodermal tumours (CNS PNETs)
British Journal of Cancer (2013)
-
The Histone Deacetylase Inhibitor Sodium Butyrate Promotes Cell Death and Differentiation and Reduces Neurosphere Formation in Human Medulloblastoma Cells
Molecular Neurobiology (2013)