Abstract
Although iron overload is clinically significant, only limited data have been published on iron overload in haematological diseases. We investigated cardiac and liver iron accumulation by magnetic resonance imaging (MRI) in a cohort of 87 subjects who did not receive chelation, including 59 haematological patients. M-HIC (MRI-based hepatic iron concentration, normal values <36 μmol/g) is a non-invasive, liver biopsy-calibrated method to analyse iron concentration. This method, calibrated to R2 (transverse relaxation rate), was used as a reference standard (M-HIC(R2)). Transfusions and ferritin were evaluated. Mean M-HIC(R2) and cardiac R* of all patients were 142 μmol/g (95% CI, 114–170) and 36.4 1/s (95% CI, 34.2–38.5), respectively. M-HIC(R2) was higher in haematological patients than in patients with chronic liver disease or normal controls (P<0.001). Clearly elevated cardiac R2* was found in two myelodysplastic syndrome (MDS) patients with severe liver iron overload. A poor correlation was found between liver and cardiac iron (n=82, r=0.322, P=0.003), in contrast to a stronger correlation in MDS (n=7, r=0.905, P=0.005). In addition to transfusions, MDS seemed to be an independent factor in iron accumulation. In conclusion, the risk for cardiac iron overload in haematological diseases other than MDS is very low, despite the frequently found liver iron overload.
Similar content being viewed by others
Introduction
While cardiac complications secondary to transfusional iron overload might be clinically significant in several haematological diseases,1 the impact of iron overload has been best characterised in thalassaemia. Accumulation of cardiac iron has been the leading cause of mortality in thalassaemia major.2 In myelodysplastic syndrome (MDS) only limited data of myocardial iron deposition have been published,3 but an adverse relationship between serum ferritin levels and overall survival has been reported.4, 5 Based on these results, transfusion need has been proposed to be included in the new adverse prognostic factors in WHO classification-based prognostic scoring system.6 In addition, iron accumulation has been found in chronic liver disease and viral hepatitis with an association of increased risk for severe infections.7
There is a clinical need for a quantitative, safe and non-invasive tool for iron measurement in specific tissues, in order to prevent toxic sequels of iron accumulation.8 As liver iron concentration measurement from biopsies is invasive and serum ferritin is nonspecific,9, 10 magnetic resonance imaging (MRI) has been used increasingly to provide reliable, quantitative iron concentration measurement.8 Recent technical and methodological developments have offered validated and feasible methods, based on proton transverse relaxation time (T2 and T2*) and its reciprocal relaxation rate (R2 and R2*), to assess iron concentration in liver11, 12, 13, 14, 15 and in myocardium.16, 17, 18 The liver R2 shows respective sensitivity and specificity of 0.85–0.94 and 0.92–1.00 to predict liver iron concentration at different thresholds,12 and cardiac T2* shows good interstudy reproducibility (coefficient of variation) of 2.3–9.3%,17, 18 and consistently good interscanner19 and intercenter20, 21 reproducibility. T2* correlates inversely with iron concentration, while R2* (1/T2*) correlates directly,3, 11, 14, 18 and cardiac R2* values ⩾50 1/s have been found to predict ventricular dysfunction.3, 16 A specific signal intensity method M-HIC (MRI-based hepatic iron concentration, μmol/g dry liver weight) was calibrated to liver biopsies (3–375 μmol/g) of 174 patients as an accurate, directly proportional method for liver iron concentration measurement. There was a mean difference of 0.8 μmol/g (95% CI, −6.3 to 7.9) between this method and the biochemical analysis.15 The interstudy variation of MRI techniques have been found to be comparable with the variation of liver biopsy.11, 12
Cardiac iron deposition has been a matter of debate in MDS. Less is known about cardiac involvement in other transfusion-dependent haematological malignancies.3, 4 A correlation between cardiac and liver iron deposition has been investigated mainly in chelated patients with thalassaemia. In these studies, no correlation3, 16, 22 but a potential causal relationship in longitudinal analysis was found.23 Other smaller studies in MDS24, 25 might have been biased by iron chelation, which alters the relationship between liver and cardiac iron, depending on the chelator regimens.3, 26, 27 Furthermore, chelation has been found to change the R2 calibration curves.28 Interestingly, two studies in non-chelated patients with MDS proposed that cardiac iron excess might occur only in the patients with the most severe liver iron overload.29, 30
The aim of this study was to investigate the relationship and frequency of cardiac and liver iron accumulation by MRI in a cohort of non-chelated patients with MDS, other haematological malignancies and chronic liver disease. Furthermore, we evaluated the number of transfused red blood cells (RBC) and plasma ferritin as iron indicators. Liver R2,11, 12 calibrated to M-HIC,14, 15 was used as the reference standard (M-HIC(R2)).
Patients and methods
Patients and controls
Patients with clinically suspected iron overload were recruited by specialists at the Department of Medicine in Turku University Hospital. A total of 87 subjects, including 75 patients and 12 healthy volunteers, were recruited in the study (Table 1). Inclusion criterion for patients, recruited by haematologists, was a clinically suspected iron overload due to a haematological disease with transfusion need. Inclusion criteria for patients with chronic liver disease, recruited by hepatologists, were decreased liver function and elevated serum ferritin levels. Exclusion criteria were iron chelation therapy and age <18 years. One phlebotomised patient was excluded from the correlations between RBC and iron load. The respective mean ages (±s.d.) of haematological patients, patients with chronic liver disease and healthy volunteers were 49.4 years (±13.8), 51.9 years (±11.5) and 39.4 years (±15.3) (P=0.044). Detailed characteristics of disease duration and transfusion dependency are presented in Table 2. All subjects were examined by MRI between May 2007 and March 2010. This prospective, single-centre study was conducted according to the Declaration of Helsinki guidelines. Written informed consent was obtained from all subjects. The study protocol was approved by the local Ethics Committee of the Hospital District of Southwest Finland.
Magnetic resonance imaging
The MRI of cardiac T2* was performed on a 1.5 T scanner (Siemens Magnetom Avanto) and liver R2 on two 1.5 T scanners (Siemens Magnetom Avanto and Siemens Magnetom Symphony, Siemens Medical Solutions, Erlangen, Germany) with the combination of body-matrix and spine-matrix surface coils. Liver imaging was obtained in 75 patients and cardiac imaging in 73 patients. One patient with MDS and one with chronic hepatopathy declined cardiac MRI. Liver and cardiac imaging was obtained from 12 normal controls. Three liver R2 values from the normal controls were missing owing to incalculable low values. The mean interval between liver and cardiac imaging was 0.25 days (range 0–8 days). Patients were instructed to fast 4 h before the MR examination.
Cardiac imaging and analysis
We used a validated T2* method first introduced by Andersson et al.16 ECG-gated breath-hold spoiled gradient recalled echo sequence (2D FLASH) was applied at different echo times (TE 3, 5, 6.5, 8, 10, 12, 14, 17, 20, 23, 26 and 29 ms) with the following parameters: repetition time 500 ms, flip angle 20°, slice thickness 10 mm, matrix size 128 × 256, field of view 350 mm, seven phase encoding steps per cardiac cycle and time of acquisition 15–25 s. In all, 47 subjects were scanned with TE of 3–29 ms and 40 subjects were scanned with TE of 5–29 ms. Minor differences in TE values had been tested for consistency in two studies.20, 21 A single short-axis mid-ventricular slice was acquired with constant imaging parameters for each subject. A black-blood preparation pulse31, 32 was applied to reduce artefacts in 79 subjects.
A region of interest of 1.0–1.3 cm2 was chosen for the signal intensity measurement from the left ventricular septum.18 All images were evaluated by an experienced reader and selected to avoid movement artefacts. A mono-exponential fitting was used in the cardiac T2*(1/R2*) measurement analysis.31, 32 R2* is directly proportional to iron concentration, and was chosen as a primary cardiac iron indicator.14 Cardiac iron overload was defined as R2*⩾50 1/s (corresponding T2*⩽20 ms), which was used as a cutoff point for normal and pathologically elevated cardiac iron.16
Liver imaging and analysis
We used a T2 multi-echo spin-echo imaging sequence with fat suppression for liver iron assessment at 11 different TE times (TE 7.2–86.4 ms, 7.2 ms interval) with the following parameters: repetition time 2000 ms, flip angle 180°, slice thickness 10 mm, matrix size 192 × 256, field of view 400 mm and time of acquisition 4.50 min. Freehand region of interest was chosen for signal intensity measurement from the right part of the right liver lobe in segments 6 and 7 by an experienced reader avoiding vessels and possible artefacts. A mono-exponential fitting with an offset constant C was used to define values of T2 and the reciprocal, R2(1/T2).11
Calibration between liver R2 and M-HIC
The R2 was calibrated to a previously in vivo and in vitro validated and biopsy-calibrated M-HIC (μmol/g liver dry weight).14, 15 As a result (Supplementary Figure 1), we were able to use M-HIC(R2) (μmol/g liver dry weight) as a reference standard and maintain the wide quantitative range of R2.11, 12 The calibration is demonstrated in Supplementary Information.
Transfusion history
The number of transfused RBC units for haematological patients was collected via a transfusion database Trace Line (MAK-SYSTEM, Paris, France), and patient reviews. The data were collected from 1996, when the database was founded. Transfusion dependency duration, transfusion dependency at the time of MRI and RBC units received at the dependency period were evaluated. In addition, four variables were analysed to evaluate the influence of these variables on transfusional iron accumulation in the liver. These were: total cumulative number of RBC units received (RBC); number of high-dose transfusion sessions (HDS); number of high-dose RBC units received (HDU); and MDS. The HDS was defined as the number of transfusion sessions in which at least four RBC units were transfused. The HDU was defined as the number of cumulative RBC units of more than two transfused in a single session. The RBC, HDS and HDU were evaluated as quantitative variables, and MDS as a qualitative variable. These variables were chosen based on an empiric evaluation of the whole transfusion data in order to investigate if a high dose of RBC units received in one session or MDS itself had an impact on iron accumulation in the liver.
Ferritin, CRP and ALT
Plasma concentration of ferritin (μg/l) was measured electrochemically (MEIA), C-reactive protein (CRP, mg/l) with immunoturbidimetric assay and alanine transaminase (ALT, U/l) according to IFCC recommendation on Roche Modular PPEE analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The median interval between blood tests and MRI was 18 days (95% CI, 18.4–30.0; range 0–126 days).
Statistical analysis
Statistical tests were performed using GraphPad Prism ver. 5, Instat ver. 3.06 (GraphPad Software, Inc., San Diego, CA, USA) and SAS/STAT ver. 9.2 of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA).
The correlations were obtained using Pearson correlation and scatter plots with least square measures linear regression analysis. Two-tailed t-test and one-way ANOVA with Tukey–Kramer multiple comparisons posttest was used between the groups. The hepatic iron concentration of haematological patients was analysed using univariate and backward linear regression analysis with RBC, HDS, HDU and MDS (exclusion criteria, P<0.10), the normality of residuals was tested with Shapiro–Wilks test, and collinearity diagnostics were performed. A receiver–operator characteristic curve was applied to search the optimal thresholds, with specificity (%), sensitivity (%), positive predictive value (PPV, %) and negative predictive value (NPV, %), and to test accuracy (area under curve (AUC)) of iron indicators. Statistical significance was defined as P<0.05.
Results
Cardiac and liver iron overload
Liver iron overload (M-HIC(R2)⩾36 μmol/g) was frequent, compared with cardiac iron overload (R2*⩾50 1/s) (Table 1). Liver iron overload was found in the majority of haematological patients (49/59, 83%) and in patients with chronic liver disease (8/15, 53%). Only three haematological patients showed cardiac iron overload. None of the patients with chronic liver disease had cardiac iron overload and none of the normal controls had either cardiac or liver iron overload.
The mean liver M-HIC(R2) was higher in haematological patients than in patients with chronic liver disease or normal controls, with respective mean values of 162 μmol/g (95% CI, 129–196), 66 μmol/g (95% CI, 33–100) and 15 μmol/g (95% CI, 7–24) (P<0.001). Haematological patients showed higher plasma ferritin than patients with chronic liver disease with respective mean values of 2060 μg/l (95% CI, 1620–2500) and 943 μg/l (95% CI, 405–1480) (P=0.020). Cardiac R2* did not differ significantly between haematological patients, patients with chronic liver disease and normal controls with respective mean values of 37 1/s (95% CI, 34–39), 36 1/s (95% CI, 33–39) and 34 1/s (95% CI, 31–37) (P=0.714).
Clinical characteristics and iron overload in haematological patients
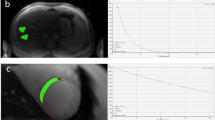
In haematological patients, clinical characteristics and hepatic and cardiac iron load are presented in Table 2. Only disease duration reached statistical significance among the mean differences of the subgroups and in the multiple comparison test between acute lymphatic leukaemia (ALL) and myelofibrosis (P<0.05). Disease duration correlated weakly with liver iron (r=0.290, P=0.026), and did not correlate with cardiac iron (r=0.130, P=0.368). Transfusion dependency duration correlated moderately with total RBC (r=0.5621, P<0.001) and liver iron (r=0.6383, P<0.001), but not with cardiac iron (r=0.2203, P=0.0965). Patients with MDS showed the highest mean cardiac and liver iron levels. Clearly elevated cardiac R2* was found in only two patients with MDS (R2*=85 and 59 1/s). A 20-year-old patient with precursor-B-cell ALL showed a borderline cardiac iron level (R2*=52 1/s). His disease history was not unusual, with respective disease and transfusion durations of 831 and 81 days. The patient had a normal echocardiography, tested negative for haemochromatosis and had no other comorbidities. Examples of iron accumulation in different patients are illustrated by cardiac MR images (Figures 1a–c).
Cardiac short-axis left ventricular MR images of three patients with similar parameters (TE=8 ms). The images illustrate the different degrees of hepatic and cardiac iron accumulation, which is seen as a signal drop. A signal intensity of myocardium was measured with constant region of interest (oval line) from MR images in each patient. Subphrenic liver, marked with an asterisk, is situated at the lower part of the image. (a) A patient with ALL; normal level of cardiac R2* (30 1/s) with only slightly elevated hepatic iron concentration (40 μmol/g). There is no visible signal drop found in the liver or the heart. (b) A patient with AML; cardiac R2* (37 1/s) was normal, although there was heavy hepatic iron overload (380 μmol/g). The liver signal dropped owing to the severe iron overload, giving more contrast to the normal cardiac tissue. (c) A patient with MDS; pathologically low R2* (85 1/s), indicating cardiac iron overload with simultaneous severe hepatic iron overload (488 μmol/g). The figure shows the signal drop of both cardiac and hepatic tissue due to excess iron.
Correlation between cardiac and liver iron
In MDS, there was a highly significant correlation (Figure 2a) between hepatic and cardiac iron overload (n=7, r=0.905, P=0.005). In the other diagnostic subgroups of aplastic anemia, acute lymphatic leukaemia, acute myeloid leukaemia, chronic leukaemia, lymphomas and chronic liver disease, no statistically significant correlation was found between hepatic and cardiac iron (P>0.43). The only exception was the group of patients with myelofibrosis, where the correlation reached statistical significance (n=6, r=0.863, P=0.027). In all subjects, the correlation (Figure 2b) was weak but significant (n=82, r=0.322, P=0.003).
A correlation between cardiac R2* and hepatic iron concentration. (a) In MDS, the cardiac iron (R2*) increases as hepatic iron concentrations increase, showing a strong dependence. Two of the MDS patients had the highest R2* values among the entire cohort. (b) All subjects (n=82) showed a weak dependence between cardiac and hepatic iron concentrations.
The effect of transfusion indexes and MDS on liver iron
In haematological patients, we used a backward regression analysis to evaluate the influence of the transfusion indexes (RBC, HDS and HDU) and MDS on hepatic iron accumulation. The RBC (P<0.001) and MDS (P=0.002) were the only explaining variables left in the final model. The HDT or HDU had no impact on the hepatic iron accumulation from transfusions (P>0.32). This model with a linear fit suggested that MDS is an independent variable that increases the transfusional liver iron overload with an average of 79 μmol/g compared with non-MDS patients.
RBC and ferritin as iron indicators in haematological patients
Hepatic iron concentration correlated significantly with both RBC and plasma ferritin (Figures 3a and b). The correlation with RBC was higher (n=58, r=0.840, P<0.001) than with ferritin (n=55. r=0.676, P<0.001). In all patients, the correlation with ferritin remained significant (r=0.713, P<0.001). The RBC was found to be more accurate than ferritin in predicting liver iron overload, as shown in receiver–operator characteristic analysis. The respective optimal threshold values for RBC and ferritin to predict iron overload were 13 RBC units (AUC=0.940, 100% specificity, 87.5% sensitivity, 100% PPV and 62.5% NPV) and 1019 μg/l (AUC=0.843, 80% specificity, 81% sensitivity, 93% PPV and 55% NPV). Cardiac iron did not correlate with RBC (n=57, r=0.100, P=0.457), and the correlation with ferritin was weak (n=54, r=0.366, P=0.007).
In haematological patients, both transfusion load (RBC) and ferritin can be used to estimate iron concentrations (HICest). The number of RBC units was more accurate, with better correlations in estimating liver iron concentration with linear fit. (a) The correlation between hepatic iron concentration and RBC (range 0–144) is demonstrated with the equation HICest(transfusion)=4.6RBC+2.1. (b) The correlation between hepatic iron concentration and ferritin is demonstrated with the equation HICest(ferritin)=0.12ferritin−77.
ALT, CRP and age
Mean ALT was at the same level in patients with haematological and chronic liver disease (P=0.959); the respective mean values were 65 U/l (95% CI, 46–84) and 66 U/l (95% CI, 11–122). The respective mean CRP of patients with haematological and liver disease were 7.1 mg/l (95% CI, 4.6–9.6) and 61.5 mg/l (95% CI, 78.4–201.4) (P=0.004). CRP did not correlate with ferritin (n=54, r=−0.041, P=0.768). ALT, CRP or patient age did not correlate with hepatic iron concentration (P=0.192, 0.700 and 0.088, respectively). Ages correlated poorly with cardiac iron (r=0.314, P=0.007).
Discussion
Liver and cardiac iron overload in the whole study cohort
In our study, cardiac iron overload was a rarity, despite the high frequency of liver iron overload, which was a cardinal feature in RBC transfused haematological malignancies. In general, iron load in haematological malignancies was higher than in chronic liver disease. We found a poor correlation between cardiac and hepatic iron accumulation in all patients, which is in agreement with other cross-sectional studies, mostly done in thalassaemia.16, 22, 33 In our cohort, this reflected the low frequency of cardiac iron overload. We can conclude that in haematological patients other than MDS, the risk of elevated cardiac iron is very low, which is a novel finding in adults.33
Liver and cardiac iron overload in haematological patients
We found liver iron overload in a large variety of haematological malignancies due to transfused units of RBC, which correlated strongly with liver iron. In contrast, RBC showed no correlation with the infrequently found cardiac iron load. There were no distinct differences found in the mean transfusion characteristics among the diagnostic subgroups (Table 2). Only distinct feature was the large proportion of transfusion-dependent patients in both MDS and myelofibrosis at the time of MRI, which was to be expected. The correlation between transfusion dependency duration and liver iron was moderate. Apparently, this can be explained by the fact that dependency duration reflected the number of transfused RBC. The frequently found hepatic iron overload and the level of iron concentration were in agreement with the results of a previous study with a similar cohort as ours.34 To our knowledge, cardiac R2* has not been studied earlier in a variety of adult haematological malignancies, but our R2* level for normal controls was generally consistent with the results of previous studies.16, 17, 19 In our cohort, clearly elevated cardiac iron was found in only two MDS patients. This principally agrees with earlier results in MDS, although there has been some inconsistency in the results.3, 4 Jensen et al.29 found elevated cardiac iron in 9/12 non-chelated patients with MDS, using a different method for cardiac iron measurement than we used. Di Tucci et al.30 found 3/22, Chacko et al.25 1/11 and Konen et al.24 1/10 patients with cardiac iron overload measured with the T2* method similar to ours.
Liver and cardiac iron overload in patients with MDS
In patients with MDS, both cardiac and liver iron overload were more pronounced than in any other subgroups. Our results suggest that iron load was increased owing to both RBC transfusions and MDS itself. The transfusion characteristics, high-volume transfusions (HDS or HDU) or patient age could not explain this result. These findings in MDS can be explained with ineffective erythropoiesis, which may lead to already increased iron overload before transfusions begin. Apparently, the high erythropoietic activity can lead to downregulation of hepcidin and paradoxically increased iron absorption, even when body iron levels are already elevated.4 Especially MDS patients with isolated erythroid dysplasia and low risk of leukaemic evolution are more likely to develop parenchymal iron overload and organ damage.35
In our study, both of the MDS patients with severe liver iron overload (>269 μmol/g)12 also had cardiac iron overload. Interestingly, this threshold for elevated cardiac iron was not found in any other haematological malignancies, despite of the severe iron overload. This result for MDS agrees with a previously detected threshold of 269 μmol/g for greatly increased risk for cardiac disease and early death.12 Similarly, Jensen et al.29 and Di Tucci et al.30 found a critical level of liver iron concentration in non-chelated MDS patients, after which cardiac iron overload occurs, although they used different methodologies than we used and failed to demonstrate correlations. Thus, our results together with the earlier findings suggest that there is a critical level of liver iron concentration for predicting cardiac iron overload in MDS. The correlation we found, between hepatic and cardiac iron in MDS and in myelofibrosis, was stronger than previously reported in patients with chelation treatment.24, 25 In addition, there have been suggestions of a longitudinal causal relationship between liver and cardiac iron accumulation in chelated patients with thalassaemia.23 Our results do not discount this possibility, but a causal relationship may be more apparent in chelated non-MDS patients. By excluding the chelated patients, we were able to explore the natural course of the iron accumulation at a selected cross-sectional time point and observe good correlation between liver and cardiac iron overload in patients with MDS and myelofibrosis.
Clinical relevance of liver iron measurement by quantitative MRI and iron indicators
The quantitative range of the M-HIC(R2) in this study (5–546 μmol/g) covered the clinically significant liver iron concentration range from normal values beyond severe liver iron overload. A total of 71 μmol/g is considered a threshold for screening haemochromatosis, in which progressive iron accumulation potentially generates fibrosis.13 In transfusional iron overload, values from 57 to 125 μmol/g are suggested for the limits of optimal chelation therapy, after which iron-induced complications increases.12 The calibration curve of our method between the R2 (Wood et al.11 and St Pierre et al.12) and M-HIC14 (Supplementary Figure 1) enabled this wide range of concentrations, based on R2 analysis.
The iron indicators of both RBC and ferritin were able to estimate M-HIC(R2), but the accuracy of RBC was higher in our cohort. Although the dependence of both of these indicators did not reach the level of quantitative MRI methods described in the Supplementary Information, we did find respective thresholds for RBC and ferritin to detect liver iron overload (13 RBC units and 1019 μg/l) with high PPV. One previous study in patients after allogeneic stem cell transplantation by Rose et al.34 also found significant correlations for both indicators. The ferritin samples in our cohort were collected at the time when CRP level was low, so ferritin was not compromised by the effects of allogeneic transplantation or infection. Indeed, infectious or inflammatory conditions were found to weaken the correlation between ferritin and liver iron in a cohort resembling ours.10 In clinical practice, we suggest that RBC is a more reliable iron indicator in haematological patients, if patients with chelation treatment or phlebotomy are excluded. However, some precautions should be taken regarding MDS, where transfusions may be responsible for only a part of the iron load.4 Thus, there remains an indication for ferritin measurement as well.
Limitations of the study
We were able to show an increased risk of MDS patients for iron overload with a relatively small cohort. The variation in the number of patients in the diagnostic subgroups may have had some influence on the results, for example, in the transfusion characteristics of the haematological subgroups. Thus, larger studies in patients with MDS are still needed to confirm our findings. Moreover, transfusion history was collected from the year the database was founded. The vast majority of the patients were diagnosed after this, and there should be no major bias in RBC collection, which is seen in the strong correlation. Nevertheless, this limitation together with the MDS feature of ‘intrinsic’ iron accumulation might had some influence in the relatively low cutoff value of 13 RBC units to predict liver iron overload. Third, the control group was ∼10 years younger than the patients. However, this should not have influenced the results, because in an earlier study in our unit a similar group of volunteers did not show any correlation between ages and liver iron concentration measured by T2 (1/R2).36 Finally, no direct invasive measurements of tissue concentrations were available. However, the MRI methods we used are justified as widely validated, non-invasive indicators for iron concentration.11, 12, 21 In cardiac imaging, we used black-blood preparation pulse in the majority of subjects as this technique has been found to be comparable to the more conventional technique used in minor extent in this study.31
Conclusion
In conclusion, the risk for cardiac iron overload in patients with haematological disease other than MDS is very low, despite the frequent finding of liver iron overload. Patients with MDS were more susceptible than other patients to both cardiac and hepatic iron overload. Cardiac and hepatic iron correlated significantly in patients with MDS and myelofibrosis. A larger study of non-chelated patients would be needed to explore these results further. Both transfused RBC units and ferritin have certain limitations, but can be used as semi-quantitative indicators of liver iron.
References
Schafer AI, Cheron RG, Dluhy R, Cooper B, Gleason RE, Soeldner JS et al. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med 1981; 304: 319–324.
Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med 1994; 331: 574–578.
Wood JC . Cardiac iron across different transfusion-dependent diseases. Blood Rev 2008; 22 (Suppl 2): S14–S21.
Fenaux P, Rose C . Impact of iron overload in myelodysplastic syndromes. Blood Rev 2009; 23 (Suppl 1): S15–S19.
Malcovati L, Della Porta MG, Cazzola M . Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica 2006; 91: 1588–1590.
Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 2007; 25: 3503–3510.
Bonkovsky HL, Banner BF, Rothman AL . Iron and chronic viral hepatitis. Hepatology 1997; 25: 759–768.
Brittenham GM, Badman DG . Noninvasive measurement of iron: report of an NIDDK workshop. Blood 2003; 101: 15–19.
Nielsen P, Günther U, Dürken M, Fischer R, Düllmann J . Serum ferritin iron in iron overload and liver damage: correlation to body iron stores and diagnostic relevance. J Lab Clin Med 2000; 135: 413–418.
Olthof AW, Sijens PE, Kreeftenberg HG, Kappert P, Irwan R, van der Jagt EJ et al. Correlation between serum ferritin levels and liver iron concentration determined by MR imaging: impact of hematologic disease and inflammation. Magn Reson Imaging 2007; 25: 228–231.
Wood JC, Enriquez C, Ghugre N, Tyzka MJ, Carson S, Nelson MD et al. MRI R2 and R2* mapping accurately estimates hepatic iron concentration in transfusion-dependent thalassemia and sickle cell disease patients. Blood 2005; 106: 1460–1465.
St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK et al. Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 2005; 105: 855–861.
Alústiza JM, Castiella A, De Juan MD, Emparanza JI, Artetxe J, Uranga M . Iron overload in the liver diagnostic and quantification. Eur J Radiol 2007; 61: 499–506.
Virtanen JM, Komu ME, Parkkola RK . Quantitative liver iron measurement by magnetic resonance imaging: in vitro and in vivo assessment of the liver to muscle signal intensity and the R2* methods. Magn Reson Imaging 2008; 26: 1175–1182.
Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V et al. Non-invasive assessment of hepatic iron stores by MRI. Lancet 2004; 363: 357–362.
Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001; 22: 2171–2179.
Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B et al. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging 2003; 18: 33–39.
Ghugre NR, Enriquez CM, Gonzalez I, Nelson MD, Coates TD, Wood JC . MRI detects myocardial iron in the human heart. Magn Reson Med 2006; 56: 681–686.
Westwood MA, Anderson LJ, Firmin DN, Gatehouse PD, Lorenz CH, Wonke B et al. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging 2003; 18: 616–620.
Westwood MA, Firmin DN, Gildo M, Renzo G, Stathis G, Markissia K et al. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemia. Int J Cardiovasc Imaging 2005; 21: 531–538.
Tanner MA, He T, Westwood MA, Firmin DN, Pennell DJ . Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica 2006; 91: 1388–1391.
Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD . Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood 2004; 103: 1934–1936.
Noetzli LJ, Carson SM, Nord AS, Coates TD, Wood JC . Longitudinal analysis of heart and liver iron in thalassemia major. Blood 2008; 112: 2973–2978.
Konen E, Ghoti H, Goitein O, Winder A, Kushnir T, Eshet Y et al. No evidence for myocardial iron overload in multitransfused patients with myelodysplastic syndrome using cardiac magnetic resonance T2 technique. Am J Hematol 2007; 82: 1013–1016.
Chacko J, Pennell DJ, Tanner MA, Hamblin TJ, Wonke B, Levy T et al. Myocardial iron loading by magnetic resonance imaging T2* in good prognostic myelodysplastic syndrome patients on long-term blood transfusions. Br J Haematol 2007; 138: 587–593.
Berdoukas V, Chouliaras G, Moraitis P, Zannikos K, Berdoussi E, Ladis V . The efficacy of iron chelator regimes in reducing cardiac and hepatic iron in patients with thalassaemia major: a clinical observational study. J Cardiovasc Magn Reson 2009; 11: 20.
Anderson LJ, Wonke B, Prescott E, Holden S, Walker JM, Pennell DJ . Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in beta-thalassaemia. Lancet 2002; 360: 516–520.
Wood JC, Aguilar M, Otto-Duessel M, Nick H, Nelson MD, Moats R . Influence of iron chelation on R1 and R2 calibration curves in gerbil liver and heart. Magn Reson Med 2008; 60: 82–89.
Jensen PD, Jensen FT, Christensen T, Eiskjaer H, Baandrup U, Nielsen JL . Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication of close relation between myocardial iron content and chelatable iron pool. Blood 2003; 101: 4632–4639.
Di Tucci AA, Matta G, Deplano S, Gabbas A, Depau C, Derudas D et al. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica 2008; 93: 1385–1388.
He T, Gatehouse PD, Kirk P, Tanner MA, Smith GC, Keegan J et al. Black-blood T2* technique for myocardial iron measurement in thalassemia. J Magn Reson Imaging 2007; 25: 1205–1209.
He T, Gatehouse PD, Smith GC, Mohiaddin RH, Pennell DJ, Firmin DN . Myocardial T2* measurements in iron-overloaded thalassemia: an in vivo study to investigate optimal methods of quantification. Magn Reson Med 2008; 60: 1082–1089.
Leung AW, Chu WC, Lam WW, Lee V, Li CK . Magnetic resonance imaging assessment of cardiac and liver iron load in transfusion dependent patients. Pediatr Blood Cancer 2009; 53: 1054–1059.
Rose C, Ernst O, Hecquet B, Maboudou P, Renom P, Noel MP et al. Quantification by magnetic resonance imaging and liver consequences of post-transfusional iron overload alone in long term survivors after allogeneic hematopoietic stem cell transplantation (HSCT). Haematologica 2007; 92: 850–853.
Cazzola M, Della Porta MG, Malcovati L . Clinical relevance of anemia and transfusion iron overload in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program 2008; 2008: 166–175 (Review).
Salo S, Alanen A, Leino R, Bondestam S, Komu M . The effect of haemosiderosis and blood transfusions on the T2 relaxation time and 1/T2 relaxation rate of liver tissue. Br J Radiol 2002; 75: 24–27.
Acknowledgements
We are grateful to M.Sc. Saija Hurme for her excellent guidance in biostatistics. This work was supported by grants from Turku University Hospital, Turku, Finland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on Blood Cancer Journal website
Supplementary information
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Virtanen, J., Remes, K., Itälä-Remes, M. et al. The relationship between cardiac and liver iron evaluated by MR imaging in haematological malignancies and chronic liver disease. Blood Cancer Journal 2, e49 (2012). https://doi.org/10.1038/bcj.2011.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bcj.2011.48
Keywords
This article is cited by
-
MRI for Quantification of Liver and Cardiac Iron in Thalassemia Major Patients: Pilot Study in Indian Population
The Indian Journal of Pediatrics (2017)