Abstract
Background:
The urokinase plasminogen activator (uPA) system is one of the best-investigated protease systems, both under physiological and pathological conditions, including various types of cancer. However, effects of co-expression of members of the uPA system in soft-tissue sarcoma (STS) patients at the protein level in both tumour tissue and serum have not been investigated yet.
Methods:
We examined 82 STS patients for protein levels of uPA, PAI-1and uPAR in tumour tissue and serum by ELISA.
Results:
A significant correlation between high antigen levels of uPA, PAI-1 or uPAR in tumour tissue, and of uPAR in serum, with poor outcome of STS patients was found for the first time. Most strikingly, we observed an additive effect of combined uPA, PAI-1 or uPAR levels in tumour tissue extracts with uPAR levels in serum on patients’ prognosis. High uPA/uPAR, PAI-1/uPAR and uPAR/uPAR antigen levels in tumour tissue/serum were associated with a 5.9-fold, 5.8-fold and 6.2-fold increased risk of tumour-related death (P=0.003, 0.001 and 0.002, respectively) compared with those patients who displayed low levels of the respective marker combination.
Conclusion:
As expression of members of the uPA system in tumour tissue and serum is additively correlated with prognosis of STS patients, our results suggest that combinations of these biomarkers can identify STS patients with a higher risk of tumour-related death.
Similar content being viewed by others
Main
The urokinase plasminogen activator (uPA) system comprises the serine protease uPA, its receptor uPAR and two inhibitors PAI-1 and PAI-2 (Duffy, 2004). Components of the uPA system have an important role in tumourigenesis, extracellular matrix (ECM) degradation, angiogenesis, as well as in proliferation, migration and adhesion of tumour cells (Duffy and Duggan, 2004; Mondino and Blasi, 2004; Pillay et al, 2007). They are prognostic factors in different types of cancer. For example, elevated tumour tissue levels of uPA have prognostic impact in a variety of cancers such as breast, colon, oesophagus, ovary and stomach cancer, high antigen levels of uPAR are associated with poor prognosis in cancer of the breast and colon, and elevated levels of PAI-1 are correlated with shortened overall and/or disease-free survival in renal, ovarian and breast cancer (Duffy and Duggan, 2004; Clark et al, 2008). Nevertheless, the clinical finding that an enzyme inhibitor does not have a protective function but is an indicator of worse prognosis is, at first glance, surprising. However, apart from being a uPA inhibitor, it has been demonstrated that PAI-1 has different, additional tumour-supporting functions (Duffy et al, 2008). Only one study has so far determined the protein expression of components of the uPA system and evaluated its impact on prognosis for soft-tissue sarcoma (STS) patients. Increasing uPA protein levels in tumour tissue were associated with local recurrence and metastasis in 69 STS patients (Choong et al, 1996). Up to now, however, there are no studies that have investigated protein levels of all three components of the uPA system in tumour tissue and serum of STS patients. Therefore, we determined the expression of uPA, uPAR and PAI-1 on protein level in a cohort of 82 adult STS patients, and evaluated their relationship with relevant clinicopathological parameters and overall survival (OS). In addition, the effect of combined uPA, uPAR and PAI-1 values in tumour tissue and serum of STS patients was analysed.
Materials and methods
Patients and tumour material
This study was performed on tumour tissue samples of 82 adult patients with histologically verified STS that have been described in previous studies (Würl et al, 2002). The study adhered to national regulations on ethical issues and was approved by the local ethical committee. All patients gave written informed consent (Department of Surgery 1, University of Leipzig, Germany). The median age of patients at surgery was 55.8 years (range 17–83 years). The median follow-up time of patients was 46 months (range 2–146 months after primary tumour resection). Tumours were staged according to the UICC system. Relevant data on clinical parameters of the STS patients are shown in Table 1.
Out of 82 STS patients, uPA, uPAR and PAI-1 antigen levels could be determined in tumour tissue samples for 80 patients, and for 79 patients in preoperative serum samples. For 77 patients, uPA, uPAR and PAI-1 protein levels were determined in both tumour tissue and serum.
Determination of uPA, uPAR and PAI-1 antigen by ELISA
Tissue extracts were prepared from frozen STS in the presence of Triton X-100 (detergent extracts) as previously described (Jänicke et al, 1994; Luther et al, 1996). Briefly, after solubilisation of membrane-bound proteins using Tris buffer containing the non-ionic detergent Triton X-100 (1%), cell debris was separated by centrifugation and the supernatant was stored at −20°C until use. For each STS patient, serum samples were obtained 2 days before surgery and were stored at −80°C until assayed. The uPA, uPAR and PAI-1 antigen content levels in tissue extracts, as well as in serum of STS patients, were determined applying commercially available ELISA kits (IMUBIND uPA ELISA # 894, IMUBIND uPAR ELISA #893 and IMUBIND PAI-1 ELISA 821; American Diagnostica Inc., Stamford, CT, USA) according to the manufacturer's instructions. Antigen concentrations in tissue extracts were expressed in ng analyte per mg of total protein.
Statistical analysis
The levels of significance between continuous variables of biological markers were calculated using Spearman's rank correlation (rs). The relationship of biological marker expression levels with clinicopathological parameters was evaluated using non-parametric Mann–Whitney or Kruskal–Wallis tests. For survival analyses, the OS of STS patients was used as follow-up end point. The association between biological marker levels and prognosis was evaluated using univariate Kaplan–Meier analyses, and the log-rank test was applied to test for differences. For multivariate analyses, Cox's proportional hazard regression model was used to calculate the relative risk and its 95% confidence interval (CI) in the analysis of OS. Multivariate models were adjusted for known clinical prognostic factors in STS patients: tumour stage, tumour type, type of tumour resection and tumour localisation. All calculations were performed using the SPSS 17.0 program (SPSS-Science, Chicago, IL, USA). All P-values were two-sided and P<0.05 was considered statistically significant.
Results
Correlation of uPA, uPAR and PAI-1 levels in tumour tissue and in serum of STS patients
We investigated the correlation between antigen levels of uPA, uPAR and PAI-1 in tumour tissue extracts (uPA-T, uPAR-T and PAI-1-T, respectively) and in serum (uPA-S, uPAR-S and PAI-1-S, respectively). In tumour tissue extracts, we detected a strong correlation between uPA-T and uPAR-T (rs=0.84, P<0.001) or PAI-1-T (rs=0.69, P<0.001), and between uPAR-T and PAI-1-T (rs=0.83, P<0.001) antigen levels (Table 2). In serum, a moderate correlation was observed between uPA-S and uPAR-S (rs=0.54, P<0.001). However, PAI-1-S values were only weakly, if at all, correlated with uPA-S (rs=0.24, P<0.05) or uPAR-S (rs=0.19, n.s.) values. Next, we evaluated the relationship between uPA, uPAR and PAI-1 antigen levels in tumour tissue extracts with that in serum (Table 2). We found a moderate, significant correlation between uPAR-S concentration and uPA-T, uPAR-T and PAI-1-T levels (rs=0.49, 0.54 and 0.46, respectively, all P<0.001). Furthermore, the uPA-S concentration was weakly but significantly correlated with uPA-T, uPAR-T and PAI-1-T values (rs=0.36, 0.38 and 0.31, respectively, all P<0.01). These results suggest that uPA, uPAR and PAI-1 levels in the tumour may affect each other or are regulated in a concerted manner and that they are strikingly related to the serum levels of uPAR protein in STS patients.
uPA, uPAR and PAI-1 antigen levels in STS tissues and association with clinical parameters and prognosis
The amount of uPA-T, uPAR-T and PAI-1-T protein has been determined in 80 STS tissue samples and was related to the whole protein content in each sample. The median protein expression for uPA-T was 1.78 ng mg−1 (range: 0–22.76), for uPAR-T 3.98 ng mg−1 (range: 0.17–103.47) and for PAI-1-T, it was 21.43 ng mg−1 (range: 0.57–1,279.0). For statistical analysis, the median values were used as cutoff points to separate STS patients into groups with low or high antigen levels in tumour tissue extracts. The association of uPA-T, uPAR-T and PAI-1-T levels with relevant clinicopathological factors is summarised in Table 1. High levels of uPA-T, uPAR-T and PAI-1-T antigen were significantly associated with histological subtype (P<0.001, P<0.001 and P=0.006, respectively), with tumour grade (P<0.001, P<0.001 and P=0.001, respectively), and with tumour stage (P<0.001, P<0.001 and P=0.006, respectively). Moreover, uPA-T, uPAR-T and PAI-1-T antigen levels were significantly higher in patients who died during follow-up time (Table 1).
For survival analysis, the Kaplan–Meier test was performed to study the effect of uPA-T, uPAR-T and PAI-1-T antigen levels on prognosis. Overall survival was significantly different between patients’ groups with high or low antigen levels for all three markers. Patients with high vs low expression of uPA-T survived on an average for 44 months vs 86 months (P=0.003), patients with high vs low expression of uPAR-T survived on an average for 54 vs 76 months (P=0.033) and those with high vs low expression of PAI-1-T survived on an average for 53 vs 79 months (P=0.004) (Table 3). The independent relationship of uPA-T, uPAR-T and PAI-1-T was studied using multivariate Cox's regression analysis. For patients whose tumours expressed either a high uPA-T or a high PAI-1-T antigen level, we detected a significantly, nearly three-fold increased risk of tumour-related death (RR=2.9, 95% CI=1.1–7.7, P=0.032; and RR=2.6, 95% CI=1.1–6.0, P=0.029, respectively) compared with those patients who displayed low uPA-T or PAI-1-T values in their tumours, respectively (Table 3). On the other hand, uPAR-T levels did not significantly contribute to the base model for OS (Table 3). Therefore, only high uPA-T or PAI-1-T antigen tumour tissue levels are independent prognostic factors for OS of STS patients.
uPA, uPAR and PAI-1 antigen concentration in serum of STS patients and association with clinical parameters and prognosis
The concentration of uPA (uPA-S), uPAR (uPAR-S) and PAI-1 (PAI-1-S) antigen in pre-operative serum samples of 79 STS patients has been measured by ELISA. We observed a median antigen concentration of 0.66 ng ml−1 (range: 0–4.76) for uPA-S, of 1.60 ng ml−1 (range: 0.24–8.03) for uPAR-S and of 1084.0 ng ml−1 (range: 44.0–6,068.5) for PAI-1-S. For statistical analysis, the median values uPA-S, uPAR-S and PAI-1-S were used as cutoff points to separate STS patients in groups with low or high antigen concentrations in serum. High concentrations of uPAR-S antigen were significantly associated with histological subtype (P=0.004), tumour grade (P<0.001) and tumour stage (P=0.019) (Table 1). On the contrary, uPA-S and PAI-1-S serum antigen levels did not show any association with clinicopathological features (Table 1).
In Kaplan–Meier analysis, a significantly different OS has been found between groups of STS patients with high or low uPAR-S concentrations. Patients with high vs low levels of uPAR-S survived on an average for 45 months vs 86 months (P=0.005) (Table 3). Serum levels of uPA and PAI-1 antigen were not associated with prognosis of STS patients in univariate analysis (Table 3). In multivariate Cox's regression analysis, we found that STS patients with elevated uPAR-S antigen levels possessed a 3.5-fold increased risk of tumour-related death (RR=3.5, 95% CI=1.5–8.3, P=0.004) compared with patients with low uPAR-S concentrations (Table 3).
Combined analysis of uPA, uPAR and PAI-1 antigen levels for OS
We assessed whether a combination of biological markers might add prognostic information for patients’ survival. For this analysis, the patient cohort was divided into four groups on the basis of the combination of high and low marker values. In Kaplan–Meier analysis, a co-detection of high levels of uPA-T and PAI-1-T or uPAR-T, and of PAI-1-T and uPAR-T, was significantly associated with a shorter OS compared with patients with low antigen levels of the combined markers in tumour tissue (Table 4). Patients with high vs low values of uPA-T/uPAR-T survived on an average for 41 vs 75 months (P=0.007), those with high vs low expression of uPA-T/PAI-1-T survived on an average for 46 vs 88 months (P=0.004), and those with high vs low expression of PAI-1-T/uPAR-T survived on an average for 53 vs 86 months (P=0.014) (Table 4). In multivariate Cox's regression analysis, the subgroup of patients with high uPA-T/uPAR-T, high uPA-T/PAI-1-T or high uPAR-T/PAI-1-T antigen levels showed the worst OS with RR values of 3.3 (95% CI=1.2–9.6, P=0.026) 3.6 (95% CI=1.2–10.4, P=0.019) or 3.2 (95% CI=1.1–9.4, P=0.034), respectively, compared with patients having tumours with low values for both markers (Table 4).
Furthermore, the effect of uPA, PAI-1 or uPAR antigen levels in tumour tissue, in combination with the uPAR antigen concentration in serum on OS, was studied. In univariate Kaplan–Meier analysis, OS was significantly different between patient subgroups, with high values of combined markers vs low values for all three marker combinations, i.e., patients with high vs low levels of uPA-T/uPAR-S survived on an average for 44 months vs 94 months (P=0.012), those with high vs low levels of uPAR-T/uPAR-S on an average for 38 vs 80 months (P=0.008) and those patients with high vs low levels of PAI-1-T/uPAR-S on an average for 39 vs 82 months (P=0.007) (Table 4).
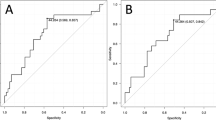
In multivariate Cox's regression analysis, we found for the subgroups of patients with a high uPA-T/uPAR-S, a high uPAR-T/uPAR-S or a high PAI-1-T/uPAR-S, an ∼6-fold significantly increased risk of tumour-related death (RR=5.9, 95% CI=1.8–19.2, P=0.003; RR=6.2, 95% CI=1.9–20.0, P=0.002; RR=5.8, 95% CI=1.9–17.0, P=0.001, respectively) compared with those patients who showed low values for each marker combination (Table 4, Figure 1). Thus, there is an additional effect on prognosis when uPA, PAI-1 and uPAR levels in tumour tissue were combined, which is even more pronounced for the combination of uPA, PAI-1 and uPAR tissue levels with uPAR serum values.
Multivariate Cox's regression hazard analysis: association of combined marker levels with overall survival of STS patients. (A) Combined values of uPAR antigen in serum and uPA antigen in tumour tissue extracts. (B) Combined values of uPAR antigen in serum and PAI-1 antigen in tumour tissue extracts. (C) Combined values of uPAR antigen in serum and in tumour tissue extracts. Patients with high expression of uPAR antigen levels in serum and with high levels of uPA (A), PAI-1 (B) or uPAR (C) in tumour tissue extracts possessed a 5.9-, 5.8- or 6.2-fold increased risk of tumour-related death compared with patients who showed a low concentration for both proteins.
Discussion
The uPA system is one of the best-investigated protease systems under both physiological and pathological conditions, including various types of cancer (Duffy et al, 2008).
There are many studies investigating the clinical impact of expression of members of the uPA system and its correlation to prognosis in carcinoma (Duffy and Duggan 2004; McMahon and Kwaan, 2007–08; Duffy et al. 2008) but so far only one study has been conducted for STS patients (Choong et al, 1996). Choong et al (1996) detected an association of increasing uPA protein levels in tumour tissue with local recurrence and metastasis in 69 STS patients. In our study, single protein levels of uPA and PAI-1, and combined protein levels of uPA, uPAR and PAI-1, in tumour tissues were significantly correlated with an up to 3.6-fold increased risk of tumour-related death. There are two main reasons for a clinical impact of expression of uPA, uPAR and PAI-1 in tumour tissues and their correlation with prognosis. First, the uPA system has a role in modulating cell adhesion, overcoming ECM boundaries and can interact with potential oncogenes. The binding of uPA to membrane-bound uPAR results in focusing active uPA to cells, and in efficient cell-associated cleavage of plasminogen to plasmin, which subsequently breaks down ECM and facilitates cancer invasion (Clark et al, 2008). On one hand, uPAR concentrates uPA enzymatic activity to the tumour cell, on the other, there is also a mutual cooperation between uPAR and further interactors: the activity of integrins, chemokines, cytokines and growth factor receptors (Ragno, 2006; Tang and Wei, 2008). Furthermore, in cancer cells, Ras signalling is linked to the uPA system (Silberman et al, 1997). Second, the uPA system has a role in overcoming tissue boundaries. Recently, a physiological role for uPAR signalling in the regulation of kidney permeability has been described (Wei et al, 2008). Another study investigating the entry of fibrosarcoma cells into the vasculature (i.e., intravasation) points to the same direction. After inhibition of uPA by natural or synthetic inhibitors in the chorioallantoic membrane of chick embryos, both inhibitors reduced intravasation and metastasis. The authors suggest uPA activation as a key step in tumour progression (Madsen et al, 2006). The effects of members of the uPA system on tumour cell biology, cell migration and metastases can be reversed in in vivo models. Mice with a targeted deficiency for uPA or PAI-1 showed a significantly reduced tumour growth after transplantation of fibrosarcoma cells. Tumours in uPA−/− and PAI-1−/− mice displayed lower proliferative and higher apoptotic indices and displayed different neovascular morphology, as compared with WT mice (Gutierrez et al, 2000). In line with these results are findings in a mouse osteosarcoma model. In this in vivo model of tibial tumours, uPAR mRNA was expressed early (4 days), whereas uPA and PAI-1 mRNA increased as the tumour invaded the surrounding tissue (3 weeks). Interestingly, there was a preferential co-localisation of uPA, uPAR and PAI-1 mRNA to the advancing front of tibial tumours (Fisher et al, 2001). Furthermore, injection of an antisense uPAR inhibitor resulted in a significant reduction in tumour volumes and in total inhibition of pulmonary metastases (Dass et al, 2005). In another mouse model, treatment with a uPAR antibody and a recombinant pigment epithelium-derived factor that may internalise uPA/uPAR complexes led to decreased ostoeosarcoma growth and metastasis (Dass and Choong 2008).
A few studies have analysed uPA and PAI-1 antigen levels in plasma/serum and its contribution to prognosis in carcinoma patients but none have been conducted for sarcoma patients (Strojan et al, 1998; Miyake et al, 1999; Abendstein et al, 2000; Rha et al, 2000; Shariat et al, 2007; Iwadate et al, 2008; Herszényi et al, 2008). Strikingly, in our study, uPAR antigen levels in serum of STS patients were found to be highly and significantly associated with poor OS in Kaplan–Meier analyses and in multivariate Cox's regression analyses. This is in line with other studies that reported that high serum levels of soluble uPAR (suPAR) were significantly associated with worse survival in colorectal, prostate, ovarian and breast cancer, as well as in multiple myeloma (Sier et al, 1998; Brünner et al, 1999; Miyake et al, 1999; Stephens et al, 1999; Riisbro et al, 2002; Rigolin et al, 2003; Shariat et al, 2007) In contrast to cell-bound uPAR, which focuses uPA mediated plasmin formation to the cell surface, the role and source of suPAR remain to be clarified (Brünner et al, 1999). Holst-Hansen et al (1999) demonstrated that the amount of suPAR released from breast cancer cell lines was directly correlated to the number of viable cells. In addition, using a breast cancer xenograft tumour model, the authors demonstrated that the concentration of suPAR in plasma was highly correlated with tumour volume. Furthermore, Shariat et al (2007) found a direct association between high suPAR levels in serum and tumour burden in prostate cancer, and its decrease after tumour removal. Overall, these studies suggest that local expression/production of uPAR on tumour cells may significantly contribute to the increased levels of suPAR levels in serum of cancer patients. In our study, we observed a relatively high correlation between uPAR antigen levels in tumour tissue and serum (rs=0.54), which may indicate that suPAR levels, at least partially, derive from cancer cells. In a recent study, Riisbro et al (2002) did not find a correlation between uPAR levels in breast cancer tissue with that in serum of breast cancer patients, and whereas uPAR levels in serum were significantly associated with worse prognosis of breast cancer patients, uPAR levels in tumour cytosols were not. However, Meng et al (2006) reported that the uPAR gene status – contributing to uPAR overexpression – in breast cancer cells from blood and tumour tissue is concordant. Therefore, besides cell-bound uPAR that is shed from primary tumour tissue, other sources of suPAR should also be considered, such as blood monocytes or neutrophile granulocytes that may become activated due a systemic reaction to tumour growth/progression (Shariat et al, 2007), or as an association with circulating tumour cells (Mustjoki et al, 2000). Correlation of uPAR levels in serum with prognosis of STS patients could improve cancer detection and monitoring of cancer progression, as investigation of serum samples is more easily performed than that of cancer tissues, which is limited by tumour size, tumour heterogeneity and freezing capacities.
Most strikingly, combined high levels of uPA, uPAR and PAI-1 in tumour tissue and of uPAR in serum were found to be correlated with an additive negative effect on prognosis. However, further possibilities of affecting tumour spread and formation of metastases could be the role of the uPA system in haemostasis, as well as in inflammatory and immune processes (Mondino and Blasi, 2004). Finally, the uPA system might facilitate the recruitment of tumour cells and tumour-associated cells at the sites of metastasis using these processes.
In conclusion, co-detection of a high expression level of these uPA system members in tumour tissue and of uPAR in serum is significantly correlated with a shortened OS of STS patients, suggesting that protein expression in tumour tissue and in serum should be considered together for prognostic evaluation.
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abendstein B, Daxenbichler G, Windbichler G, Zeimet AG, Geurts A, Sweep F, Marth C (2000) Predictive value of uPA, PAI-1, HER-2 and VEGF in the serum of ovarian cancer patients. Anticancer Res 20: 569–572
Brünner N, Nielsen HJ, Hamers M, Christensen IJ, Thorlacius-Ussing O, Stephens RW (1999) The urokinase plasminogen activator receptor in blood from healthy individuals and patients with cancer. APMIS 107: 160–167
Choong PF, Fernö M, Akerman M, Willén H, Långström E, Gustafson P, Alvegård T, Rydholm A (1996) Urokinase-plasminogen-activator levels and prognosis in 69 soft-tissue sarcomas. Int J Cancer 69: 268–272
Clark JC, Dass CR, Choong PF (2008) A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol 134: 281–297
Dass CR, Choong PF (2008) uPAR mediates anticancer activity of PEDF. Cancer Biol Ther 7: 1262–1270
Dass CR, Nadesapillai AP, Robin D, Howard ML, Fisher JL, Zhou H, Choong PF. (2005) Downregulation of uPAR confirms link in growth and metastasis of osteosarcoma. Clin Exp Metastasis 22: 643–652
Duffy MJ (2004) The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des 10: 39–49
Duffy MJ, Duggan C (2004) The urokinase plasminogen activator system: a rich source of tumour markers for the individualised management of patients with cancer. Clin Biochem 37: 541–548
Duffy MJ, McGowan PM, Gallagher WM (2008) Cancer invasion and metastasis: changing views. J Pathol 214: 283–293
Fisher JL, Mackie PS, Howard ML, Zhou H, Choong PF (2001) The expression of the urokinase plasminogen activator system in metastatic murine osteosarcoma: an in vivo mouse model. Clin Cancer Res 7: 1654–1660
Gutierrez LS, Schulman A, Brito-Robinson T, Noria F, Ploplis VA, Castellino FJ (2000) Tumor development is retarded in mice lacking the gene for urokinase-type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res 60: 5839–5847
Herszényi L, Farinati F, Cardin R, István G, Molnár LD, Hritz I, De Paoli M, Plebani M, Tulassay Z (2008) Tumour marker utility and prognostic relevance of cathepsin B, cathepsin L, urokinase-type plasminogen activator, plasminogen activator inhibitor type-1, CEA and CA 19-9 in colorectal cancer. BMC Cancer 8: 194
Holst-Hansen C, Hamers MJ, Johannessen BE, Brünner N, Stephens RW (1999) Soluble urokinase receptor released from human carcinoma cells: a plasma parameter for xenograft tumour studies. Br J Cancer 81: 203–211
Iwadate Y, Hayama M, Adachi A, Matsutani T, Nagai Y, Hiwasa T, Saeki N (2008) High serum level of plasminogen activator inhibitor-1 predicts histological grade of intracerebral gliomas. Anticancer Res 28: 415–418
Jänicke F, Pache L, Schmitt M, Ulm K, Thomssen C, Prechtl A, Graeff H (1994) Both the cytosols and detergent extracts of breast cancer tissues are suited to evaluate the prognostic impact of the urokinase-type plasminogen activator and its inhibitor, plasminogen activator inhibitor type 1. Cancer Res 54: 2527–2530
Luther T, Flössel C, Albrecht S, Kotzsch M, Müller M (1996) Tissue factor expression in normal and abnormal mammary gland. Nat Med 2: 491–492
Madsen MA, Deryugina EI, Niessen S, Cravatt BF, Quigley JP (2006) Activity-based protein profiling implicates urokinase activation as a key step in human fibrosarcoma intravasation. J Biol Chem 281: 15997–16005
McMahon B, Kwaan HC (2007–08) The plasminigen activator system and cancer. Pathophysiol Haemost Thromb 36: 184–194
Meng S, Tripathy D, Shete S, Ashfaq R, Saboorian H, Haley B, Frenkel E, Euhus D, Leitch M, Osborne C, Clifford E, Perkins S, Beitsch P, Khan A, Morrison L, Herlyn D, Terstappen LW, Lane N, Wang J, Uhr J (2006) uPAR and HER-2 gene status in individual breast cancer cells from blood and tissue. PNAS 46: 17361–17365
Miyake H, Hara I, Yamanaka K, Gohji K, Arakawa S, Kamidono S (1999) Elevation of serum levels of urokinase-type plasminogen activator and its receptor is associated with disease progression and prognosis in patients with prostate cancer. Prostate 39: 123–129
Mondino A, Blasi F (2004) uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol 25: 450–455
Mustjoki S, Sidenius N, Sier CF, Blasi F, Elonen E, Alitalo R, Vaheri A (2000) Soluble urokinase receptor levels correlate with number of circulating tumor cells in acute myeloid leukemia and decrease rapidly during chemotherapy. Cancer Res 60: 7126–7132
Pillay V, Dass CR, Choong PF (2007) The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol 25: 33–39
Ragno P (2006) The urokinase receptor: a ligand or a receptor? Story of a sociable molecule. Cell Mol Life Sci 63: 1028–1037
Rha SY, Yang WI, Gong SJ, Kim JJ, Yoo NC, Roh JK, Min JS, Lee KS, Kim BS, Chung HC (2000) Correlation of tissue and blood plasminogen activation system in breast cancer. Cancer Lett 150: 137–145
Rigolin GM, Tieghi A, Ciccone M, Bragotti LZ, Cavazzini F, Della Porta M, Castagnari B, Carroccia R, Guerra G, Cuneo A, Castoldi G (2003) Soluble urokinase-type plasminogen activator receptor (suPAR) as an independent factor predicting worse prognosis and extra-bone marrow involvement in multiple myeloma patients. Br J Haematol 120: 953–959
Riisbro R, Christensen IJ, Piironen T, Greenall M, Larsen B, Stephens RW, Han C, Høyer-Hansen G, Smith K, Brünner N, Harris AL (2002) Prognostic significance of soluble urokinase plasminogen activator receptor in serum and cytosol of tumor tissue from patients with primary breast cancer. Clin Cancer Res 8: 1132–1141
Shariat SF, Roehrborn CG, McConnell JD, Park S, Alam N, Wheeler TM, Slawin KM (2007) Association of the circulating levels of the urokinase system of plasminogen activation with the presence of prostate cancer and invasion, progression, and metastasis. J Clin Oncol 25: 349–355
Sier CF, Stephens R, Bizik J, Mariani A, Bassan M, Pedersen N, Frigerio L, Ferrari A, Danø K, Brünner N, Blasi F (1998) The level of urokinase-type plasminogen activator receptor is increased in serum of ovarian cancer patients. Cancer Res 58: 1843–1849
Silberman S, Janulis M, Schultz RM (1997) Characterization of downstream Ras signals that induce alternative protease-dependent invasive phenotypes. J Biol Chem 272: 5927–5935
Stephens RW, Nielsen HJ, Christensen IJ, Thorlacius-Ussing O, Sørensen S, Danø K, Brünner N. (1999) Plasma urokinase receptor levels in patients with colorectal cancer: relationship to prognosis. J Natl Cancer Inst 91: 869–874
Strojan P, Budihna M, Smid L, Vrhovec I, Skrk J (1998) Urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (PAI-1) in tissue and serum of head and neck squamous cell carcinoma patients. Eur J Cancer 34: 1193–1197
Tang CH, Wei Y (2008) The urokinase receptor and integrins in cancer progression. Cell Mol Life Sci 65: 1916–1932
Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J (2008) Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63
Würl P, Kappler M, Meye A, Bartel F, Köhler T, Lautenschläger C, Bache M, Schmidt H, Taubert H (2002) Co-expression of survivin and TERT and risk of tumour-related death in patients with soft-tissue sarcoma. Lancet 359: 943–945
Acknowledgements
We thank Antje Zobjack for her excellent technical assistance. HT was supported by a grant from the Deutsche Krebshilfe No. 107590 and Wilhelm-Roux-Program of BMBF/NBL3 (FKZ: 19/31). VM and MK were partially supported by a grant from the Deutsche Krebshilfe No. 107965. The work was associated to the German Sarcoma Study Group K.O.S.A.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Taubert, H., Würl, P., Greither, T. et al. Co-detection of members of the urokinase plasminogen activator system in tumour tissue and serum correlates with a poor prognosis for soft-tissue sarcoma patients. Br J Cancer 102, 731–737 (2010). https://doi.org/10.1038/sj.bjc.6605520
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6605520
Keywords
This article is cited by
-
Do coagulation or fibrinolysis reflect the disease condition in patients with soft tissue sarcoma?
BMC Cancer (2022)
-
Significance of coagulation and fibrinolysis markers for benign and malignant soft tissue tumors
BMC Cancer (2021)
-
Expression of urokinase-type plasminogen activator system in non-metastatic prostate cancer
World Journal of Urology (2020)
-
Elevated levels of serum urokinase plasminogen activator predict poor prognosis in hepatocellular carcinoma after resection
BMC Cancer (2019)
-
The prognostic relevance of urokinase-type plasminogen activator (uPA) in the blood of patients with metastatic breast cancer
Scientific Reports (2019)