News & Views |
Featured
-
-
Article |
 All-cis 1,2,3,4,5,6-hexafluorocyclohexane is a facially polarized cyclohexane
All-cis 1,2,3,4,5,6-hexafluorocyclohexane is a facially polarized cyclohexane
The highest-energy stereoisomer of 1,2,3,4,5,6-hexafluorocyclohexane, with all the fluorines ‘up’, has been prepared in a 12-step protocol. The molecule adopts a chair conformation with three triaxial C–F bonds on one face generating a polarized ring. In the solid state the molecules pack in an orientation consistent with electrostatic ordering.
- Neil S. Keddie
- , Alexandra M. Z. Slawin
- & David O'Hagan
-
Article |
 Chiral self-sorting and amplification in isotropic liquids of achiral molecules
Chiral self-sorting and amplification in isotropic liquids of achiral molecules
The spontaneous resolution of racemic mixtures can occur when the molecules are confined in a crystal lattice, on surfaces or in other well-ordered assemblies. Now, mirror symmetry breaking within an isotropic liquid of achiral molecules has been observed. These liquids show strong chiral amplification and provide a possible mode of emergence of chirality in prebiotic fluids.
- Christian Dressel
- , Tino Reppe
- & Carsten Tschierske
-
Article |
 Design and synthesis of the first triply twisted Möbius annulene
Design and synthesis of the first triply twisted Möbius annulene
Most cyclic conjugated molecules, such as benzene, exhibit two sides. Möbius annulenes, however, with an odd number of 180° twists in their π system, are one-sided and violate the Hückel rule. Now, using a topological trick it is demonstrated that triply twisted systems are not particularly strained and probably easier to synthesize than singly twisted ones.
- Gaston R. Schaller
- , Filip Topić
- & Rainer Herges
-
Article |
 Switchable enantioseparation based on macromolecular memory of a helical polyacetylene in the solid state
Switchable enantioseparation based on macromolecular memory of a helical polyacetylene in the solid state
Reversible chirality switching and memory is demonstrated in a helical polyacetylene. Both the helicity of the polymer backbone and the axial chirality of the side chains contribute to the memory effect. When used to produce a chiral stationary phase for a chromatographic enantiomer resolution it was possible to switch the elution order under identical chromatographic conditions.
- Kouhei Shimomura
- , Tomoyuki Ikai
- & Katsuhiro Maeda
-
-
Article |
 End-to-end conformational communication through a synthetic purinergic receptor by ligand-induced helicity switching
End-to-end conformational communication through a synthetic purinergic receptor by ligand-induced helicity switching
Biological receptors communicate information through ligand-induced conformational changes. A synthetic receptor with a boron-containing binding site that can selectively and reversibly complex a ligand (such as a purine nucleoside) is shown to function in a similar fashion. The resulting conformational change is relayed through the receptor, communicating structural information about the ligand to a spectroscopic reporter more than 2 nm away.
- Robert A. Brown
- , Vincent Diemer
- & Jonathan Clayden
-
Article |
 Phase-transfer-catalysed asymmetric synthesis of tetrasubstituted allenes
Phase-transfer-catalysed asymmetric synthesis of tetrasubstituted allenes
Substituted allenes with axial chirality are of great utility in organic chemistry owing to their unique structure and reactivity, but synthetic methods to access them are limited. Here, a catalytic asymmetric synthesis of tetrasubstituted allenes is described that builds on the use of phase-transfer-catalysed asymmetric functionalization of 1-alkylallene-1,3-dicarboxylates.
- Takuya Hashimoto
- , Kazuki Sakata
- & Keiji Maruoka
-
News & Views |
 Reflections on stereochemistry
Reflections on stereochemistry
No longer held in Bürgenstock or the preserve of stereochemists, the Bürgenstock conference on stereochemistry is much more than its name suggests. The diverse range of subjects discussed at the meeting highlights the fundamental importance of chemistry in other scientific disciplines ranging from molecular biology to materials science.
- Stuart J. Conway
-
Article |
 Highly enantioselective trapping of zwitterionic intermediates by imines
Highly enantioselective trapping of zwitterionic intermediates by imines
Reactions with unstable and highly reactive zwitterionic intermediates generated in transition-metal-catalysed processes provide new opportunities for molecular constructions. Here imines, activated by chiral organocatalysts, have been employed to trap the zwitterionic intermediates to give polyfunctionalized indole and oxindole derivatives in a single step with excellent diastereoselectivity and enantioselectivity.
- Huang Qiu
- , Ming Li
- & Wen-Hao Hu
-
Review Article |
 The progression of chiral anions from concepts to applications in asymmetric catalysis
The progression of chiral anions from concepts to applications in asymmetric catalysis
Novel concepts in asymmetric catalysis have the potential to open up previously inaccessible reaction space. This Review reflects on the origins of an area that has undergone dramatic recent advancement: the use of chiral anions in asymmetric catalysis. Details of a selection of the latest examples are also given.
- Robert J. Phipps
- , Gregory L. Hamilton
- & F. Dean Toste
-
Article |
 Dissecting the mechanisms of a class of chemical glycosylation using primary 13C kinetic isotope effects
Dissecting the mechanisms of a class of chemical glycosylation using primary 13C kinetic isotope effects
Chemical glycosylations are perhaps the most important reactions in glycoscience, but the mechanisms are not well understood. Here, quantum chemical calculations combined with natural-abundance NMR measurements of 13C kinetic isotope effects reveal both associative and dissociative mechanisms at the extremes of a continuum that depends on the relative stereochemistry of the substrate and the anomeric configuration of the product.
- Min Huang
- , Graham E. Garrett
- & David Crich
-
Article |
 Revealing the stereospecific chemistry of the reaction of Cl with aligned CHD3(ν1 = 1)
Revealing the stereospecific chemistry of the reaction of Cl with aligned CHD3(ν1 = 1)
Steric effects are a key concept for understanding chemical reactivity. Now, by aligning reactants through control of the polarization of the infrared laser in a crossed-beam experiment, a three-dimensional view of how a reaction proceeds is reported. The results show striking dependences on the direction from which the laser-aligned reagents approach.
- Fengyan Wang
- , Kopin Liu
- & T. Peter Rakitzis
-
News & Views |
 Stripping down SN2
Stripping down SN2
The mechanism of the SN2 reaction is fundamental to understanding and controlling the stereochemistry of organic reactions, but surrounding solvent molecules may complicate the textbook picture. Micro-solvation studies have now explored the stereochemical consequences of the presence of one or two solvent molecules.
- Andrew J. Orr-Ewing
-
News & Views |
 Correlating sterics in catalysis
Correlating sterics in catalysis
Recognizing that an analogy can be drawn between steric effects in drug discovery and asymmetric catalysis has led to a powerful technique that can explain and potentially predict the outcome of asymmetric reactions.
- Scott J. Miller
-
Article |
 A gold-catalysed enantioselective Cope rearrangement of achiral 1,5-dienes
A gold-catalysed enantioselective Cope rearrangement of achiral 1,5-dienes
The Cope rearrangement has been known since the 1940s but, until now, no catalytic asymmetric variant has been reported. Here, a gold(I) catalyst is shown to induce an asymmetric Cope rearrangement of achiral 1,5-dienes containing a cyclopropylidene moiety to produce vinyl cyclopropanes in high yield and good to excellent enantioselectivities.
- Ryan J. Felix
- , Dieter Weber
- & Michel R. Gagné
-
-
Article |
 Multidimensional steric parameters in the analysis of asymmetric catalytic reactions
Multidimensional steric parameters in the analysis of asymmetric catalytic reactions
Many parameters have been designed to describe steric size, but few have been able to explain consistently the selectivity of asymmetric catalytic reactions. Here, Sterimol parameters — originally used to develop quantitative structure–activity relationships in medicinal chemistry — have been used to quantify enantioselectivity in a diverse collection of asymmetric catalytic reactions.
- Kaid C. Harper
- , Elizabeth N. Bess
- & Matthew S. Sigman
-
Article |
 Binary fluorous tagging enables the synthesis and separation of a 16-stereoisomer library of macrosphelides
Binary fluorous tagging enables the synthesis and separation of a 16-stereoisomer library of macrosphelides
A 16-member diastereoisomer library known to contain macrosphelides A and E is synthesized as a mixture with the aid of a new encoding strategy for fluorous mixture synthesis. A simple process of sequential demixing and tag removal provides each of the isomers in individual, pure form. Analysis of the other library members ultimately leads to a structural reassignment for macrosphelide D.
- Dennis P. Curran
- , Mantosh K. Sinha
- & Dae-Hyun Cho
-
-
Article |
 A biomimetic polyketide-inspired approach to small-molecule ligand discovery
A biomimetic polyketide-inspired approach to small-molecule ligand discovery
The design and synthesis of a family of chiral and conformationally constrained oligomers is described. Asymmetric synthesis of the monomers is presented and the preparation of a 160,000-member library of diverse tetramers via split-and-pool methods is discussed. From this library, a non-covalent ligand to the DNA-binding domain of p53 was discovered.
- Claudio Aquino
- , Mohosin Sarkar
- & Glenn C. Micalizio
-
Article |
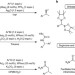 Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds
Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds
Stereoselective Suzuki–Miyaura cross-coupling reactions involving non-benzylic secondary alkylboronates are notoriously challenging. Here, an enantioselective synthesis of 1,1-diboronyl compounds using asymmetric conjugate borylation, followed by chemoselective mono cross-coupling with inversion at the diboron centre, is used to produce highly enantioenriched benzylic or allylic boronates, which themselves are useful reagents for a number of processes.
- Jack Chang Hung Lee
- , Robert McDonald
- & Dennis G. Hall
-
Article |
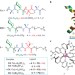 Chiral information harvesting in dendritic metallopeptides
Chiral information harvesting in dendritic metallopeptides
2,2′-bipyridine ligands coordinate to metals to form chiral propeller-like complexes. Now, this chirality is shown to be controlled by the coordination of 2,2′-bipyridine ligands that bear helical oligopeptides, which incorporate chiral amino acids at positions remote from the metal centre. This chirality is further translated, via the metal centre, to other achiral-oligopeptide-containing ligands.
- Naoki Ousaka
- , Yuki Takeyama
- & Eiji Yashima
-
-
News & Views |
 A stereochemical sojourn
A stereochemical sojourn
Stereochemistry represents a common thread uniting chemists from a range of sub-disciplines at the Bürgenstock conference, an annual scientific meeting rich in tradition and characterized by intensive, interdisciplinary discussion.
- Mark S. Taylor
-
Article |
 Stereoselective C–C bond formation catalysed by engineered carboxymethylproline synthases
Stereoselective C–C bond formation catalysed by engineered carboxymethylproline synthases
The reaction of enols and enolates with electrophiles is used extensively in synthesis. Here, protein engineering — substituting amino acid residues in an enzyme active site — is used to produce biocatalysts for the control of enolate chemistry. The adapted enzymes enable stereoselective C–C bond formation yielding N-heterocycles in high diastereomeric excess by the reaction of trisubstituted-enolates.
- Refaat B. Hamed
- , J. Ruben Gomez-Castellanos
- & Christopher J. Schofield
-
Research Highlights |
Selective soap-films
The enantiomers of chiral compounds pass through doped soap films at different rates resulting in chiral separation.
- Stephen Davey
-
News & Views |
 Breaking free of chiral symmetry
Breaking free of chiral symmetry
Helical macromolecules are ubiquitous in nature, and almost always adopt a one-handed conformation. Synthetic systems, in contrast, are typically obtained in racemic right- and left-handed mixtures. A helical phenylene oligomer has now been prepared that forms a non-racemic mixture on crystallization, and on oxidation locks one conformation in.
- Eiji Yashima
-
News & Views |
 Stereochemistry on the shores
Stereochemistry on the shores
The annual Bürgenstock conference brings together a select band of chemists to talk about the many different facets of stereochemistry, and the unique format of the meeting encourages plenty of discussion and debate alongside the traditional lectures and poster presentations.
- Paul W. Davies
-
Research Highlights |
 Stereoconvergent solution
Stereoconvergent solution
In the key step of a natural product synthesis, two diastereomers of an intermediate react with opposite stereo- and regioselectivity to provide a single product enantiomer.
- Stephen Davey
-
Article |
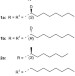 The effect of isotopic substitution on the chirality of a self-assembled helix
The effect of isotopic substitution on the chirality of a self-assembled helix
Benzene-1,3,5-tricarboxamides are known to self-assemble via intramolecular hydrogen bonding into helical columnar aggregates. Here it is shown that the introduction of a stereocentre by an isotopic substitution — replacing hydrogen for deuterium on the methylene groups next to the amide — is sufficient to direct the helicity of the formed aggregate.
- Seda Cantekin
- , Diederik W. R. Balkenende
- & E. W. Meijer
-
Article |
 Reversing the direction in a light-driven rotary molecular motor
Reversing the direction in a light-driven rotary molecular motor
Biological rotary motors can alter their mechanical function by changing the direction of rotary motion. Now, researchers have designed a synthetic light-driven rotary motor in which the direction of rotation can be reversed on command by changing the chirality of the molecular motor through base-induced epimerization.
- Nopporn Ruangsupapichat
- , Michael M. Pollard
- & Ben L. Feringa
-
Article |
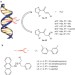 Catalytic enantioselective syn hydration of enones in water using a DNA-based catalyst
Catalytic enantioselective syn hydration of enones in water using a DNA-based catalyst
Nature frequently shows exquisite control over reactions both of water and in water. Here, the enantioselective conjugate addition of water to an enone — a reaction that has no equivalent in conventional homogeneous catalysis — is catalysed by a copper complex of an achiral ligand that is non-covalently bound to DNA.
- Arnold J. Boersma
- , David Coquière
- & Gerard Roelfes
-
Research Highlights |
 Radicals under tension
Radicals under tension
The transition state of a polymer isomerization has been stabilized under sonication-induced elongation, leading to the formation of the less stable cis-isomer.
- Anne Pichon
-
Article |
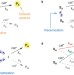 Direct enantio-convergent transformation of racemic substrates without racemization or symmetrization
Direct enantio-convergent transformation of racemic substrates without racemization or symmetrization
Processes that convert racemic chiral compounds into enantioenriched chiral products are highly sought after. Here, in a copper-catalysed borylation reaction one enantiomer of a cyclic allylic ether reacts with anti-stereoselectivity and the other reacts with syn-stereoselectivity. The starting material is not easily racemized and this new process is dubbed an enantioconvergent reaction.
- Hajime Ito
- , Shun Kunii
- & Masaya Sawamura
-
News & Views |
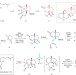 Moving backwards in new ways
Moving backwards in new ways
Pattern mapping is a synthetic tactic that is complementary to standard retrosynthetic analysis. When combined with the concept of traceless stereochemical guidance, it leads to an efficient synthesis of the steroidal natural product (±)-aplykurodinone-1.
- Nathan Collett
- & Rich G. Carter
-
Article |
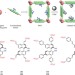 A series of isoreticular chiral metal–organic frameworks as a tunable platform for asymmetric catalysis
A series of isoreticular chiral metal–organic frameworks as a tunable platform for asymmetric catalysis
A series of chiral metal–organic frameworks with channels of tunable size have been modified through post-synthetic functionalization to bear catalytic centres along their pore walls. The resulting materials catalyse a carbon–carbon-bond-forming reaction, and the enantioselectivity of the transformation can be altered by changing the size of the channels.
- Liqing Ma
- , Joseph M. Falkowski
- & Wenbin Lin
-
Article |
 Highly enantioselective synthesis and cellular evaluation of spirooxindoles inspired by natural products
Highly enantioselective synthesis and cellular evaluation of spirooxindoles inspired by natural products
A Lewis-acid-catalysed 1,3-dipolar cycloaddition provides rapid access to a variety of substituted spirooxindoles. Initial cellular evaluations supports the view that compound collections based on natural-product-inspired scaffolds constructed with complex stereochemistry, and decorated with assorted substituents, will be a rich source of compounds with diverse bioactivity.
- Andrey P. Antonchick
- , Claas Gerding-Reimers
- & Herbert Waldmann
-
Article |
 Computational evidence that hyperconjugative interactions are not responsible for the anomeric effect
Computational evidence that hyperconjugative interactions are not responsible for the anomeric effect
The anomeric effect, which influences the position of polar substituents in the chair conformation of various heterocycles, is commonly rationalized using hyperconjugation. Now, by theoretically studying molecules that display the anomeric effect, strong evidence is provided that hyperconjugation is not responsible and it is better interpreted in terms of electrostatics.
- Yirong Mo
-
Research Highlights |
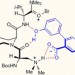 Resolving rotamers
Resolving rotamers
A peptide-catalysed bromination provides access to an important class of chiral compounds in high enantiomeric purity.
- Stephen Davey
-
Article |
 Chiral-auxiliary-mediated 1,2-cis-glycosylations for the solid-supported synthesis of a biologically important branched α-glucan
Chiral-auxiliary-mediated 1,2-cis-glycosylations for the solid-supported synthesis of a biologically important branched α-glucan
The difficulty in the stereoselective introduction of 1,2-cis-glycosides is a major stumbling block in the solid-supported synthesis of oligosaccharides. Now, this has been achieved for a biologically important glucoside containing multiple 1,2-cis-glycosidic linkages with complete anomeric control by using glycosyl donors having a participating (S)-(phenylthiomethyl)benzyl chiral auxiliary at C-2.
- Thomas J. Boltje
- , Jin-Hwan Kim
- & Geert-Jan Boons
-
Research Highlights |
 Hand-picked guests
Hand-picked guests
Metal–organic cages made with 1,1-biphenyl ligands act as hosts for enantioselective absorption.
- Neil Withers
-
Article |
 Ion-triggered spring-like motion of a double helicate accompanied by anisotropic twisting
Ion-triggered spring-like motion of a double helicate accompanied by anisotropic twisting
Helical molecules in biological systems commonly undergo extension, contraction and unidirectional twisting motions, but such twisting — promising for the construction of molecular machines — has rarely been achieved in synthetic systems. Now, a chiral double helix has been prepared whose spring-like motion is accompanied by an anisotropic twist under the control of sodium ions.
- Kazuhiro Miwa
- , Yoshio Furusho
- & Eiji Yashima
-
Article |
 Efficient stereo- and regioselective hydroxylation of alkanes catalysed by a bulky polyoxometalate
Efficient stereo- and regioselective hydroxylation of alkanes catalysed by a bulky polyoxometalate
The ability to selectively transform the C–H bonds of simple alkanes to useful functional groups such as alcohols is a key step in the move away from petrochemical feedstocks. Now, it has been shown that the oxidation of alkanes can be catalysed by a bulky polyoxometalate species using hydrogen peroxide as a stoichiometric oxidant.
- Keigo Kamata
- , Kazuhiro Yonehara
- & Noritaka Mizuno
-
Review Article |
 Induction of chiral porous solids containing only achiral building blocks
Induction of chiral porous solids containing only achiral building blocks
The synthesis or separation of chiral compounds is crucial for many areas of chemistry, with chiral solids having important roles as catalysts or separating agents. This Review covers recent progress and future avenues for developing methods of preparing chiral solids from achiral starting materials.
- Russell E. Morris
- & Xianhui Bu
-
Research Highlights |
 Racemization on the rack
Racemization on the rack
Thermally stable stereoisomers can be interconverted by the application of a mechanical force using ultrasound irradiation.
- Stephen Davey
-
News & Views |
 The same but different
The same but different
Electromerism is an unfamiliar concept to many chemists and refers to molecules that are not conventional isomers but instead differ in how the electrons are distributed across their structure. A novel example of such electromers has now been demonstrated.
- Thomas Bally
-
Article |
 Stereoinduction by distortional asymmetry
Stereoinduction by distortional asymmetry
Steric, torsion, stereoelectronic and polar effects are widely used to explain and predict the stereochemical outcome of synthetic organic reactions. Here, the asymmetric distortion of the reactant is considered and used to explain the observed stereoselectivity where these accepted models are unable to provide a clear prediction.
- Robert V. Kolakowski
- & Lawrence J. Williams
-
News & Views |
 Stereochemistry by remote control
Stereochemistry by remote control
The stereochemical lability of cycloalkylzinc reagents combined with a large difference in reactivity between epimers has been exploited to form a wide variety of interesting organic compounds with both high yields and diastereoselectivities.
- Frank Glorius
-
Article |
 Highly diastereoselective Csp3–Csp2 Negishi cross-coupling with 1,2-, 1,3- and 1,4-substituted cycloalkylzinc compounds
Highly diastereoselective Csp3–Csp2 Negishi cross-coupling with 1,2-, 1,3- and 1,4-substituted cycloalkylzinc compounds
Highly diastereoselective Negishi cross-coupling reactions between 2-, 3- and 4-substituted cycloalkylzinc reagents and aryl iodides are described. In all cases, the thermodynamically most stable diastereomers of the cross-coupling products were obtained. NMR spectroscopy and density functional theory calculations were performed in order to rationalize the observed stereoselectivities.
- Tobias Thaler
- , Benjamin Haag
- & Paul Knochel

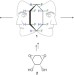 A Janus cyclohexane ring
A Janus cyclohexane ring Restricting rotation
Restricting rotation A photo finish
A photo finish Higher or lower?
Higher or lower?