Featured
-
-
Article
| Open Access Protein–lipid charge interactions control the folding of outer membrane proteins into asymmetric membranes
Protein–lipid charge interactions control the folding of outer membrane proteins into asymmetric membranes
Biological membranes are asymmetric bilayers, but little is known about how this asymmetry modulates membrane protein folding or stability. Now, folding and stability assays with bacterial outer membrane proteins reveal an exquisite sensitivity to asymmetric membrane charge distribution and a required matching of protein charge for efficient folding.
- Jonathan M. Machin
- , Antreas C. Kalli
- & Sheena E. Radford
-
Article
| Open Access Precisely patterned nanofibres made from extendable protein multiplexes
Precisely patterned nanofibres made from extendable protein multiplexes
Molecular systems with coincident cyclic and superhelical symmetry axes have considerable advantages for materials design as they can be lengthened or shortened by changing the length of the monomers. Now a systematic approach to generate modular repeat protein oligomers with combined symmetry that can be extended by repeat propagation has been developed.
- Neville P. Bethel
- , Andrew J. Borst
- & David Baker
-
News & Views |
 Polymeric protagonists for biological processes
Polymeric protagonists for biological processes
Complexity is a hallmark of biological systems, but scientific experiments are typically conducted in simplified conditions. Now, diverse polymers that mimic the local environments of complex biological mixtures have been shown to improve protein folding, stability and function.
- Alana P. Gudinas
- & Danielle J. Mai
-
Article |
 How synonymous mutations alter enzyme structure and function over long timescales
How synonymous mutations alter enzyme structure and function over long timescales
Enzymes with identical sequences of amino acids can display varying activities when encoded with mRNA with different properties, but why this is the case has been a mystery. Now, it has been shown that synonymous mutations in mRNA alter the partitioning of proteins into long-lived soluble misfolded states with varying activities.
- Yang Jiang
- , Syam Sundar Neti
- & Edward P. O’Brien
-
Article
| Open Access The ribosome stabilizes partially folded intermediates of a nascent multi-domain protein
The ribosome stabilizes partially folded intermediates of a nascent multi-domain protein
Most proteins must fold co-translationally on the ribosome to adopt biologically active conformations, yet structural, mechanistic descriptions are lacking. Using 19F NMR spectroscopy to study a nascent multi-domain protein has now enabled the identification of two co-translational folding intermediates that are significantly more stable than intermediates formed off the ribosome, suggesting that the ribosome may thermodynamically regulate folding.
- Sammy H. S. Chan
- , Tomasz Włodarski
- & John Christodoulou
-
Article |
 Conformational and functional changes of the native neuropeptide somatostatin occur in the presence of copper and amyloid-β
Conformational and functional changes of the native neuropeptide somatostatin occur in the presence of copper and amyloid-β
Metal-free amyloid-β (Aβ) and metal-bound Aβ (metal–Aβ) are found in the brain of patients with Alzheimer’s disease. Now, it has been shown that the conformation of a native neuropeptide, somatostatin, is changed in the presence of copper ions, Aβ and metal–Aβ. The conformational change results in a loss of function of somatostatin as a neurotransmitter and a gain of function as a modulator against metal–Aβ.
- Jiyeon Han
- , Jiwon Yoon
- & Mi Hee Lim
-
Article
| Open Access Interactions between nascent proteins and the ribosome surface inhibit co-translational folding
Interactions between nascent proteins and the ribosome surface inhibit co-translational folding
During polypeptide biosynthesis, a strong interaction can occur between a segment of an emerged, disordered nascent protein and the ribosomal surface. Now, it has been shown that competition between this ribosomal binding and the folding energetics of an immunoglobulin-like domain modulates the mechanism of co-translational folding.
- Anaïs M. E. Cassaignau
- , Tomasz Włodarski
- & John Christodoulou
-
Article |
 SARS-CoV-2 simulations go exascale to predict dramatic spike opening and cryptic pockets across the proteome
SARS-CoV-2 simulations go exascale to predict dramatic spike opening and cryptic pockets across the proteome
Simulations of the SARS-CoV-2 proteome that include over 0.1 s of aggregate data are reported. Spike opening was observed, revealing cryptic epitopes that differ between variants, explaining differential interactions with antibodies and receptors that determine pathogenicity. The cryptic pockets described provide new targets for antivirals and a wealth of mechanistic insight.
- Maxwell I. Zimmerman
- , Justin R. Porter
- & Gregory R. Bowman
-
Article |
 O-GlcNAc modification of small heat shock proteins enhances their anti-amyloid chaperone activity
O-GlcNAc modification of small heat shock proteins enhances their anti-amyloid chaperone activity
The post-translational modification O-GlcNAc on amyloid-forming proteins can inhibit their aggregation. Now, it has been shown that O-GlcNAc modification of small heat shock proteins HSP27, αA- and αB-crystallin can increase their anti-amyloid activity and block the amyloid formation of both α-synuclein and Aβ(1–42). A mechanism for this protective effect based on decreased physical interactions is also proposed.
- Aaron T. Balana
- , Paul M. Levine
- & Matthew R. Pratt
-
Article |
 Protein folding modulates the chemical reactivity of a Gram-positive adhesin
Protein folding modulates the chemical reactivity of a Gram-positive adhesin
Bacteria use thioester-bond-containing proteins to covalently bind to host surfaces and withstand large mechanical shocks. Now, thioester bond reactivity has been shown to be force-dependent: forces >35 pN inhibit bond cleavage by primary amine ligands, whereas forces <6 pN enable reversible reformation. This force-modulated thioester bond reactivity could potentially enable bacterial mobility and a route by which they optimize infection.
- Alvaro Alonso-Caballero
- , Daniel J. Echelman
- & Julio M. Fernandez
-
Article |
 Dynamics of oligomer populations formed during the aggregation of Alzheimer’s Aβ42 peptide
Dynamics of oligomer populations formed during the aggregation of Alzheimer’s Aβ42 peptide
Aβ42 oligomers are key toxic species associated with protein aggregation; however, the molecular pathways determining the dynamics of oligomer populations have remained unknown. Now, direct measurements of oligomer populations, coupled to theory and computer simulations, define and quantify the dynamics of Aβ42 oligomers formed during amyloid aggregation.
- Thomas C. T. Michaels
- , Andela Šarić
- & Tuomas P. J. Knowles
-
Perspective |
 Proteomimetics as protein-inspired scaffolds with defined tertiary folding patterns
Proteomimetics as protein-inspired scaffolds with defined tertiary folding patterns
The complexity of proteins has inspired chemists to seek artificial mimetics of protein structure and function. Historically, most such work has focused on analogues of small, isolated segments; however, there is growing interest in mimicry of larger, intact tertiary folds. This Perspective surveys the emerging body of work on these agents, termed ‘proteomimetics’, discusses their construction and outlines some of the remaining challenges.
- W. Seth Horne
- & Tom N. Grossmann
-
Article |
 Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation
Heteromultivalent peptide recognition by co-assembly of cyclodextrin and calixarene amphiphiles enables inhibition of amyloid fibrillation
Heteromultivalency, which involves the simultaneous interactions of more than one type of ligand with more than one type of receptor, is common in biological systems but challenging to engineer artificially. Now, a heteromultivalent platform prepared by co-assembling cyclodextrin and calixarene amphiphiles has shown self-adaptive peptide binding with high affinity. The platform was used to sequester amyloid β-peptides, reducing amyloid cytotoxicity.
- Zhe Xu
- , Shaorui Jia
- & Dong-Sheng Guo
-
Article |
 Towards simple kinetic models of functional dynamics for a kinase subfamily
Towards simple kinetic models of functional dynamics for a kinase subfamily
Molecular dynamics simulations for seven members of the Src kinase family have now revealed a conserved step-wise deactivation process, potentially druggable intermediate states, and quantitatively similar thermodynamics and kinetics across the entire family.
- Mohammad M. Sultan
- , Gert Kiss
- & Vijay S. Pande
-
Article |
 Cholesterol catalyses Aβ42 aggregation through a heterogeneous nucleation pathway in the presence of lipid membranes
Cholesterol catalyses Aβ42 aggregation through a heterogeneous nucleation pathway in the presence of lipid membranes
Cholesterol embedded in lipid membranes strongly promotes the aggregation of Aβ42 that is associated with Alzheimer's disease. Now, a kinetic analysis has shown that the mechanism of action responsible for this effect involves the introduction of a heterogeneous nucleation pathway that enhances the primary nucleation rate of Aβ42 aggregation by up to 20-fold.
- Johnny Habchi
- , Sean Chia
- & Michele Vendruscolo
-
Article |
 Distinct thermodynamic signatures of oligomer generation in the aggregation of the amyloid-β peptide
Distinct thermodynamic signatures of oligomer generation in the aggregation of the amyloid-β peptide
Mapping energy landscapes has proved to be a powerful approach for studying reaction mechanisms. Now, this strategy has been applied to determine the activation energies and entropies that characterize the molecular steps in the misfolding and aggregation of the amyloid-β peptide, revealing striking differences between the thermodynamic signatures of primary and secondary nucleation.
- Samuel I. A. Cohen
- , Risto Cukalevski
- & Sara Linse
-
Article |
 Ribosomal synthesis and folding of peptide-helical aromatic foldamer hybrids
Ribosomal synthesis and folding of peptide-helical aromatic foldamer hybrids
The extent to which peptide synthesis by the ribosome can tolerate the inclusion of non-peptidic components is not clear. Yet such hybrids would expand the range of ribosomally synthesized structures. Now it has been shown that tRNAs acylated by aromatic foldamers can initiate the ribosomal synthesis of non-cyclic and cyclic foldamer–peptide hybrid molecules. The oligo-aryl segments contain folding information that can control peptide conformation in the hybrids.
- Joseph M. Rogers
- , Sunbum Kwon
- & Ivan Huc
-
Article |
 Structure-based inhibitors of tau aggregation
Structure-based inhibitors of tau aggregation
Tau aggregation is associated with Alzheimer's disease and dozens of related dementias. Now atomic structures of the aggregation-prone segment VQIINK in repeat 2 of tau have been reported. Inhibitors designed using these structures block seeding by full-length tau better than inhibitors that target the VQIVYK aggregation segment in repeat 3.
- P. M. Seidler
- , D. R. Boyer
- & D. S. Eisenberg
-
Article |
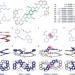 Designing cooperatively folded abiotic uni- and multimolecular helix bundles
Designing cooperatively folded abiotic uni- and multimolecular helix bundles
Rationally designed arrays of hydrogen bonds between aromatic oligoamide segments have now been shown to generate abiotic helix-turn-helix and unexpected dimeric and trimeric helix bundle motifs. These structures show kinetic and thermodynamic stability, and cooperative folding in nonpolar solvents.
- Soumen De
- , Bo Chi
- & Ivan Huc
-
Article |
 A 31-residue peptide induces aggregation of tau's microtubule-binding region in cells
A 31-residue peptide induces aggregation of tau's microtubule-binding region in cells
The self-propagation of misfolded conformations of tau occurs in neurodegenerative diseases, including Alzheimer's disease. The microtubule-binding region, tau244-372, reproduces much of the aggregation behaviour of tau in cells and animal models. Now, it has been shown that a 31-residue peptide from tau's R3 domain forms a cross-β conformation that efficiently seeds aggregation of tau244-372 in cells.
- Jan Stöhr
- , Haifan Wu
- & William F. DeGrado
-
Article |
 An infrared spectroscopy approach to follow β-sheet formation in peptide amyloid assemblies
An infrared spectroscopy approach to follow β-sheet formation in peptide amyloid assemblies
There is increasing evidence that highly dynamic, polydisperse peptide oligomers are the toxic species in amyloid-related diseases such as Alzheimer's and Parkinson's. Now, the secondary structure of individual amyloid oligomers has been analysed directly for the first time using a combination of ion-mobility spectrometry–mass spectrometry and gas-phase infrared spectroscopy.
- Jongcheol Seo
- , Waldemar Hoffmann
- & Kevin Pagel
-
Article |
 Switchable photooxygenation catalysts that sense higher-order amyloid structures
Switchable photooxygenation catalysts that sense higher-order amyloid structures
Selectively degrading the pathogenic, aggregated amyloid state of proteins, without affecting the functional state, is a potential therapeutic strategy for treating amyloid diseases. Now, photooxygenation catalysts that are active only when bound to the cross-β-sheet structure of the amyloid form have been developed.
- Atsuhiko Taniguchi
- , Yusuke Shimizu
- & Motomu Kanai
-
-
News & Views |
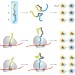 When ribosomes pick the structure
When ribosomes pick the structure
Anfinsen's principle tells us that the folded structure of a protein is determined solely by its sequence. Now, it has been shown that the rate at which a polypeptide chain is synthesized in the cell can affect which of two alternative folded structures it adopts.
- Elin M. Sivertsson
- & Laura S. Itzhaki
-
-
Article |
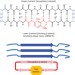 Amyloid β-sheet mimics that antagonize protein aggregation and reduce amyloid toxicity
Amyloid β-sheet mimics that antagonize protein aggregation and reduce amyloid toxicity
A family of robust β-sheet macrocycles that can display a variety of heptapeptide sequences from different amyloid proteins is introduced. These amyloid β-sheet mimics can be tailored to antagonize aggregation of the proteins, thereby reducing the toxicity associated with diseases such as Alzheimer's.
- Pin-Nan Cheng
- , Cong Liu
- & James S. Nowick
-
News & Views |
 Turbo-charged crosslinking
Turbo-charged crosslinking
The efficient production of stable bioactive proteins often requires the selective formation of several disulfide crosslinks. Two recent studies have now shown that replacing cysteine with selenocysteine in the unfolded protein can autocatalyse the formation of the desired crosslinks.
- David J. Craik
-
Article |
 Key stabilizing elements of protein structure identified through pressure and temperature perturbation of its hydrogen bond network
Key stabilizing elements of protein structure identified through pressure and temperature perturbation of its hydrogen bond network
The pressure- and temperature-dependent changes of various hydrogen bonds within ubiquitin have been determined at very high resolution using NMR H-bond scalar couplings. The measured perturbations show a correlation with the sequence separation between donor and acceptor residues, and indicate that certain topologically crucial H-bonds are specifically stabilized.
- Lydia Nisius
- & Stephan Grzesiek
-
Article |
 Protein fold determined by paramagnetic magic-angle spinning solid-state NMR spectroscopy
Protein fold determined by paramagnetic magic-angle spinning solid-state NMR spectroscopy
Despite recent progress, solving protein structures using solid-state NMR spectroscopy is not routine. Now, a method for the rapid determination of global protein fold is reported, based on measurements of 15N longitudinal paramagnetic relaxation enhancements in several protein variants modified with covalently attached cysteine–EDTA–Cu2+ tags.
- Ishita Sengupta
- , Philippe S. Nadaud
- & Christopher P. Jaroniec
-
Article |
 Ion mobility–mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation
Ion mobility–mass spectrometry reveals a conformational conversion from random assembly to β-sheet in amyloid fibril formation
Amyloid cascades leading to peptide β-sheet fibrils are central to many diseases. Intermediate assemblies were recently identified as the toxic agents, but obtaining structural details of these early oligomers has largely been unsuccessful with traditional techniques. Here, ion mobility methods provide evidence for structural transitions from random to β-sheet assembly.
- Christian Bleiholder
- , Nicholas F. Dupuis
- & Michael T. Bowers
-
News & Views |
 Interface influence
Interface influence
The aggregation of proteins into fibrils plays a crucial role in neurological conditions such as Parkinson's disease. Further insight into fibril formation has now been gained that reveals the effect of hydrophobic surfaces, including air.
- Ian W. Hamley
-
Research Highlights |
 Behold the fold
Behold the fold
Folding techniques have been used on printed titanium hydride sheets to create three-dimensional structures.
- Neil Withers

 Organic solvent enhances oxidative folding of disulfide-rich proteins
Organic solvent enhances oxidative folding of disulfide-rich proteins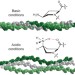 Flip to unzip
Flip to unzip Peptides do the twist
Peptides do the twist