Article
|
Open Access
Featured
-
-
Article
| Open Access TAF15 amyloid filaments in frontotemporal lobar degeneration
TAF15 amyloid filaments in frontotemporal lobar degeneration
Cryogenic electron microscopy structures of amyloid filaments extracted from patient brains reveal that the protein TAF15 forms filaments that characterize certain cases of frontotemporal lobar degeneration.
- Stephan Tetter
- , Diana Arseni
- & Benjamin Ryskeldi-Falcon
-
Article
| Open Access Predicting multiple conformations via sequence clustering and AlphaFold2
Predicting multiple conformations via sequence clustering and AlphaFold2
An analysis of the evolutionary distribution of predicted structures for the metamorphic protein KaiB using AF-Cluster reveals that both conformations of KaiB were distributed in clusters across the KaiB family.
- Hannah K. Wayment-Steele
- , Adedolapo Ojoawo
- & Dorothee Kern
-
Article
| Open Access Macromolecular condensation buffers intracellular water potential
Macromolecular condensation buffers intracellular water potential
Water thermodynamics drive changes in macromolecular assembly that rapidly restore intracellular water availability in response to physiological fluctuations in temperature, pressure and osmotic strength.
- Joseph L. Watson
- , Estere Seinkmane
- & Emmanuel Derivery
-
Article
| Open Access TDP-43 forms amyloid filaments with a distinct fold in type A FTLD-TDP
TDP-43 forms amyloid filaments with a distinct fold in type A FTLD-TDP
Cryo-electron microscopy structures and mass spectrometry analyses show that TAR DNA-binding protein 43 (TDP-43) forms amyloid filaments with a distinct fold in type A frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP) compared with TDP-43 filaments in type B FTLD-TDP and amyotrophic lateral sclerosis.
- Diana Arseni
- , Renren Chen
- & Benjamin Ryskeldi-Falcon
-
Article
| Open Access A cytosolic surveillance mechanism activates the mitochondrial UPR
A cytosolic surveillance mechanism activates the mitochondrial UPR
We identify a highly controlled cytosolic surveillance mechanism that integrates independent mitochondrial stress signals to initiate the mitochondrial unfolded protein response (UPR), revealing a link between mitochondrial and cytosolic proteostasis.
- F. X. Reymond Sutandy
- , Ines Gößner
- & Christian Münch
-
Article |
 Structural basis of BAM-mediated outer membrane β-barrel protein assembly
Structural basis of BAM-mediated outer membrane β-barrel protein assembly
The structural basis of the late-stage intermediate assembly of outer membrane β-barrel proteins mediated by the bacterial β-barrel assembly machinery is determined.
- Chongrong Shen
- , Shenghai Chang
- & Haohao Dong
-
Article
| Open Access Visualization of translation and protein biogenesis at the ER membrane
Visualization of translation and protein biogenesis at the ER membrane
Structural studies of the ribosome-associated endoplasmic reticulum translocon complex based on cryo-electron tomography and molecular modelling reveal multiple intermediate states and interactions between the components of the complex and its cofactors.
- Max Gemmer
- , Marten L. Chaillet
- & Friedrich Förster
-
Article |
 Mechanism of an intramembrane chaperone for multipass membrane proteins
Mechanism of an intramembrane chaperone for multipass membrane proteins
Biochemical and structural analysis of intermediates during multipass membrane protein biogenesis showed how an intramembrane chaperone guides nascent membrane proteins to a semi-enclosed lipid-filled cavity where they are inserted and folded correctly.
- Luka Smalinskaitė
- , Min Kyung Kim
- & Ramanujan S. Hegde
-
Article
| Open Access Substrate-driven assembly of a translocon for multipass membrane proteins
Substrate-driven assembly of a translocon for multipass membrane proteins
Biochemical reconstitution and functional analysis reveal how newly synthesized multipass membrane proteins dynamically remodel the translocon to facilitate their successful biogenesis.
- Arunkumar Sundaram
- , Melvin Yamsek
- & Robert J. Keenan
-
Article |
 Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis
Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis
Ageing alters the kinetics of translation elongation in both Caenorhabditis elegans and Saccharomyces cerevisiae.
- Kevin C. Stein
- , Fabián Morales-Polanco
- & Judith Frydman
-
Article |
 Structure of Hsp90–Hsp70–Hop–GR reveals the Hsp90 client-loading mechanism
Structure of Hsp90–Hsp70–Hop–GR reveals the Hsp90 client-loading mechanism
The cryo-electron microscopy structure of the glucocorticoid receptor (GR)-loading complex—a complex in which Hsp70 loads GR onto Hsp90 and Hop—is described, providing insights into how the chaperones Hsp90 and Hsp70 coordinate to facilitate GR remodelling for activation.
- Ray Yu-Ruei Wang
- , Chari M. Noddings
- & David A. Agard
-
Article |
 Structure of Hsp90–p23–GR reveals the Hsp90 client-remodelling mechanism
Structure of Hsp90–p23–GR reveals the Hsp90 client-remodelling mechanism
Studies based on cryo-electron microscopy structures of Hsp90 chaperone complexes reveal the molecular mechanism of the chaperone-mediated maturation of the human glucocorticoid receptor.
- Chari M. Noddings
- , Ray Yu-Ruei Wang
- & David A. Agard
-
Article |
 De novo protein design by deep network hallucination
De novo protein design by deep network hallucination
The trRosetta neural network was used to iteratively optimise model proteins from random 100-amino-acid sequences, resulting in ‘hallucinated’ proteins, which when expressed in bacteria closely resembled the model structures.
- Ivan Anishchenko
- , Samuel J. Pellock
- & David Baker
-
Article |
 DAXX represents a new type of protein-folding enabler
DAXX represents a new type of protein-folding enabler
A protein chaperone system is identified that consists of proteins with poly-Asp/Glu sequence, and may have an important role in diseases characterized by protein aggregation.
- Liangqian Huang
- , Trisha Agrawal
- & Xiaolu Yang
-
Article |
 HSP40 proteins use class-specific regulation to drive HSP70 functional diversity
HSP40 proteins use class-specific regulation to drive HSP70 functional diversity
The binding and activation of HSP70 by class B J-domain proteins is subject to an autoinhibitory regulatory mechanism that controls substrate targeting to HSP70 and is required for the disaggregation of amyloid fibres.
- Ofrah Faust
- , Meital Abayev-Avraham
- & Rina Rosenzweig
-
Article |
 Molecular dissection of amyloid disaggregation by human HSP70
Molecular dissection of amyloid disaggregation by human HSP70
The molecular steps that lead to the disaggregation of amyloid fibrils are shown to involve the synergistic action of HSP70 and its co-chaperones DNAJB1 and HSP110.
- Anne S. Wentink
- , Nadinath B. Nillegoda
- & Bernd Bukau
-
Article |
 Computational design of transmembrane pores
Computational design of transmembrane pores
An approach for the design of protein pores is demonstrated by the computational design and subsequent experimental expression of both an ion-selective and a large transmembrane pore.
- Chunfu Xu
- , Peilong Lu
- & David Baker
-
Article |
 An intramembrane chaperone complex facilitates membrane protein biogenesis
An intramembrane chaperone complex facilitates membrane protein biogenesis
The PAT complex, an intermembrane chaperone complex comprising the ER-resident membrane proteins CCDC47 and Asterix, directly interacts with nascent transmembrane domains to facilitate the biogenesis of multi-spanning membrane proteins.
- Patrick J. Chitwood
- & Ramanujan S. Hegde
-
Article |
 Extracellular proteostasis prevents aggregation during pathogenic attack
Extracellular proteostasis prevents aggregation during pathogenic attack
A systematic analysis of the proteostasis network of secreted proteins in Caenorhabditis elegans identifies numerous regulators of protein homeostasis outside the cell, and highlights the contribution of extracellular proteostasis to host defence.
- Ivan Gallotta
- , Aneet Sandhu
- & Della C. David
-
Article |
 Structure of a nascent membrane protein as it folds on the BAM complex
Structure of a nascent membrane protein as it folds on the BAM complex
Cryo-electron microscopy structures of a folding intermediate on the BAM complex of Escherichia coli reveal how interactions between the BamA catalyst and substrate permit stable association during folding, followed by rapid turnover.
- David Tomasek
- , Shaun Rawson
- & Daniel Kahne
-
Article |
 Processive extrusion of polypeptide loops by a Hsp100 disaggregase
Processive extrusion of polypeptide loops by a Hsp100 disaggregase
A combination of optical tweezers and fluorescent-particle tracking is used to dissect the dynamics of the Hsp100 disaggregase ClpB, and show that the processive extrusion of polypeptide loops is the mechanistic basis of its activity.
- Mario J. Avellaneda
- , Kamila B. Franke
- & Sander J. Tans
-
Article |
 Regulation of α-synuclein by chaperones in mammalian cells
Regulation of α-synuclein by chaperones in mammalian cells
Chaperones interact with a canonical motif in α-synuclein, which can be prevented by phosphorylation of α-synuclein at Tyr39, whereas inhibition of this interaction leads to the localization of α-synuclein to the mitochondria and aggregate formation.
- Björn M. Burmann
- , Juan A. Gerez
- & Sebastian Hiller
-
Article |
 Nitrosative stress drives heart failure with preserved ejection fraction
Nitrosative stress drives heart failure with preserved ejection fraction
iNOS-driven dysregulation of the IRE1α–XBP1 pathway leads to cardiomyocyte dysfunction in mice and recapitulates the systemic and cardiovascular features of human heart failure with preserved ejection fraction.
- Gabriele G. Schiattarella
- , Francisco Altamirano
- & Joseph A. Hill
-
Letter |
 Distinct proteostasis circuits cooperate in nuclear and cytoplasmic protein quality control
Distinct proteostasis circuits cooperate in nuclear and cytoplasmic protein quality control
Ubiquitin chains linked to cytoplasmic misfolded proteins are different from those linked to nuclear misfolded proteins, each requiring a distinct combination of molecular chaperones and ubiquitination circuitries.
- Rahul S. Samant
- , Christine M. Livingston
- & Judith Frydman
-
Letter |
 Structures of filaments from Pick’s disease reveal a novel tau protein fold
Structures of filaments from Pick’s disease reveal a novel tau protein fold
The structures of tau filaments from patients with the neurodegenerative disorder Pick’s disease show that the filament fold is different from that of the tau filaments found in Alzheimer’s disease.
- Benjamin Falcon
- , Wenjuan Zhang
- & Michel Goedert
-
Letter |
 Cotranslational assembly of protein complexes in eukaryotes revealed by ribosome profiling
Cotranslational assembly of protein complexes in eukaryotes revealed by ribosome profiling
Cotranslational assembly is a prevalent mechanism for the formation of oligomeric complexes in Saccharomyces cerevisiae, with one subunit serving as scaffold for the translation of partner subunits.
- Ayala Shiber
- , Kristina Döring
- & Bernd Bukau
-
Letter |
 A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage
A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage
Sperm-activated lysosomes enhance proteostasis in nematode oocytes just before fertilization; this could prevent transmission of damaged proteins to the next generation and may explain the immortality of the germ-cell lineage.
- K. Adam Bohnert
- & Cynthia Kenyon
-
Letter |
 Cytosolic proteostasis through importing of misfolded proteins into mitochondria
Cytosolic proteostasis through importing of misfolded proteins into mitochondria
Proteins prone to aggregation in yeast are imported into mitochondria under stress conditions, suggesting that mitochondrial import and proteolysis may help to disaggregate proteins in the cytoplasm.
- Linhao Ruan
- , Chuankai Zhou
- & Rong Li
-
Letter |
 Alternative modes of client binding enable functional plasticity of Hsp70
Alternative modes of client binding enable functional plasticity of Hsp70
Hsp70 binds unfolded protein segments in its groove, but can also bind and stabilize folded protein structures, owing to its moveable lid, with ATP hydrolysis and co-chaperones allowing control of these contrasting effects.
- Alireza Mashaghi
- , Sergey Bezrukavnikov
- & Sander J. Tans
-
Letter |
 The epichaperome is an integrated chaperome network that facilitates tumour survival
The epichaperome is an integrated chaperome network that facilitates tumour survival
Chaperomes are dynamic assemblies of proteins that regulate cellular homeostasis but specific cellular stresses remodel chaperome components into a stable chaperome network called the epichaperome, which might offer a new cancer target.
- Anna Rodina
- , Tai Wang
- & Gabriela Chiosis
-
Article |
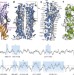 Structural basis for the antifolding activity of a molecular chaperone
Structural basis for the antifolding activity of a molecular chaperone
The solution structure of SecB, a molecular chaperone that exhibits strong antifolding activity, in complex with alkaline phosphatase and maltose-binding protein captured in their unfolded states.
- Chengdong Huang
- , Paolo Rossi
- & Charalampos G. Kalodimos
-
Letter |
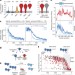 Cotranslational signal-independent SRP preloading during membrane targeting
Cotranslational signal-independent SRP preloading during membrane targeting
The signal recognition particle (SRP) preferentially binds peptides destined for secretion before peptide-targeting signals are translated through recognition of elements in their mRNA, including non-coding sequences.
- Justin W. Chartron
- , Katherine C. L. Hunt
- & Judith Frydman
-
Letter |
 Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation
Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation
Acute protein folding stress in the mitochondrial matrix activates both increased chaperone availability within the matrix and reduced matrix-localized protein synthesis through translational inhibition.
- Christian Münch
- & J. Wade Harper
-
Article |
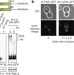 Failure of RQC machinery causes protein aggregation and proteotoxic stress
Failure of RQC machinery causes protein aggregation and proteotoxic stress
Defects in the ribosome quality control (RQC) complex, which clears proteins that stalled during translation, can cause neurodegeneration; here it is shown that in RQC-defective cells a peptide tail added by the RQC subunit 2 to stalled polypeptides promotes their aggregation and the sequestration of chaperones in these aggregates, affecting normal protein quality control processes.
- Young-Jun Choe
- , Sae-Hun Park
- & F. Ulrich Hartl
-
Article |
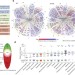 ∆F508 CFTR interactome remodelling promotes rescue of cystic fibrosis
∆F508 CFTR interactome remodelling promotes rescue of cystic fibrosis
A new deep proteomic analysis method is used to identify proteins that interact with wild-type cystic fibrosis transmembrane conductance regulator (CFTR) and its mutant version that is the major cause of cystic fibrosis.
- Sandra Pankow
- , Casimir Bamberger
- & John R. Yates III
-
Letter |
 Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation
Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation
An efficient protein disaggregation system uncovered in metazoan cells requires transient interactions between J-protein co-chaperones of classes A and B, which synergistically boost HSP70-dependent disaggregation activity, providing a flexible further level of regulation for metazoan protein quality control, with direct relevance to human diseases such as age-related neurodegeneration.
- Nadinath B. Nillegoda
- , Janine Kirstein
- & Bernd Bukau
-
Letter |
 Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch
Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch
The structural polymorphism of intrinsically disordered protein 4E-BP2 allows it to regulate translation initiation through post-translational modification-mediated folding, exemplifying a new and potentially general mechanism of biological regulation mediated by intrinsically disordered proteins.
- Alaji Bah
- , Robert M. Vernon
- & Julie D. Forman-Kay
-
Article |
 Structural basis for outer membrane lipopolysaccharide insertion
Structural basis for outer membrane lipopolysaccharide insertion
Lipopolysaccharide, an essential component of the outer membranes of Gram-negative bacteria, is inserted by LptD–LptE, a protein complex with a unique ‘barrel and plug’ architecture; the structure, molecular dynamics simulations and functional assays of the LptD–LptE complex of Salmonella typhimurium suggest that lipopolysaccharide may pass through the barrel and is then inserted into the outer leaflet of the outer membrane through a lateral opening between two β-strands of LptD.
- Haohao Dong
- , Quanju Xiang
- & Changjiang Dong
-
Letter |
 The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress
The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress
Molecular, pharmacological and functional data show that haematopoietic stem cells (HSCs) are predisposed to ER-stress-mediated apoptosis compared to closely related progenitors; a framework for understanding how stress signalling is coordinated within the hematopoietic hierarchy and integrated with stemness is provided, and may have implications for the improvement of clinical transplantation of HSCs.
- Peter van Galen
- , Antonija Kreso
- & John E. Dick
-
Letter |
 Single-molecule fluorescence probes dynamics of barrier crossing
Single-molecule fluorescence probes dynamics of barrier crossing
Here the Kramers diffusion coefficient and free-energy barrier are characterized for the first time through single-molecule fluorescence measurements of the temperature- and viscosity-dependence of the transition path time for protein folding.
- Hoi Sung Chung
- & William A. Eaton
-
Article |
 Structural insight into the biogenesis of β-barrel membrane proteins
Structural insight into the biogenesis of β-barrel membrane proteins
The crystal structure of BamA, the central component of the β-barrel assembly machinery (BAM) complex, from N. gonorrhoeae and H. ducreyi is determined; the structure consists of an interior cavity capped by extracellular loops, an exterior rim with a narrowed hydrophobic surface and a lateral opening of the barrel domain, providing insight into a possible route for the insertion of β-barrel membrane proteins into the bacterial outer membrane.
- Nicholas Noinaj
- , Adam J. Kuszak
- & Susan K. Buchanan
-
Letter |
 The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein
The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein
Biochemical and structural investigation of a model for prion-induced neurodegeneration—antibody binding to PrPC—reveals the role of the PrP flexible tail and reactive oxygen species in mediating toxicity.
- Tiziana Sonati
- , Regina R. Reimann
- & Adriano Aguzzi
-
Letter |
 Reshaping of the conformational search of a protein by the chaperone trigger factor
Reshaping of the conformational search of a protein by the chaperone trigger factor
The bacterial chaperone named trigger factor is found to stabilize protein folding intermediates that eventually convert to the native state, suggesting that chaperones play a direct role in instructing protein folding.
- Alireza Mashaghi
- , Günter Kramer
- & Sander J. Tans
-
Letter |
 Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26
Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26
MERS-CoV is a newly emerged coronavirus that is related to SARS-CoV and has proven fatal in half of the people it has infected to date: here the crystal structure of the MERS-CoV receptor binding domain is presented in complex with its receptor on human cells, CD26.
- Guangwen Lu
- , Yawei Hu
- & George F. Gao
-
News |
 Misfolded protein transmits Parkinson’s from cell to cell
Misfolded protein transmits Parkinson’s from cell to cell
Link between cell death and protein clumps opens pathway to possible treatment.
- Virginia Hughes
-
Letter |
 Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-β
Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-β
It is shown that the formation of amyloid-β oligomers, one of the histopathological signatures of Alzheimer’s disease, can be triggered by small quantities of a specifically truncated and post-translationally modified version of amyloid-β.
- Justin M. Nussbaum
- , Stephan Schilling
- & George S. Bloom
-
Letter |
 Differential positioning of adherens junctions is associated with initiation of epithelial folding
Differential positioning of adherens junctions is associated with initiation of epithelial folding
Shifts in the position of adherens junctions, triggered by a change in the ratio of Bazooka and Par-1, initiate epithelial folding in the Drosophila embryo.
- Yu-Chiun Wang
- , Zia Khan
- & Eric F. Wieschaus
-
News Feature |
 Prions and chaperones: Outside the fold
Prions and chaperones: Outside the fold
Susan Lindquist has challenged conventional thinking on how misfolded proteins drive disease and may power evolution. But she still finds that criticism stings.
- Bijal P. Trivedi
-
Letter |
 Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy
Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy
Aneuploidy is shown to be induced by pleiotropic stress conditions (especially Hsp90 inhbition) in yeast, leading to stress adaptation.
- Guangbo Chen
- , William D. Bradford
- & Rong Li

 The UFM1 E3 ligase recognizes and releases 60S ribosomes from ER translocons
The UFM1 E3 ligase recognizes and releases 60S ribosomes from ER translocons