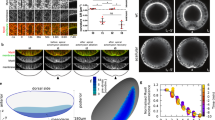
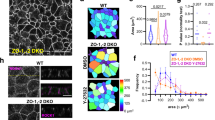
Abstract
During tissue morphogenesis, simple epithelial sheets undergo folding to form complex structures. The prevailing model underlying epithelial folding involves cell shape changes driven by myosin-dependent apical constriction1. Here we describe an alternative mechanism that requires differential positioning of adherens junctions controlled by modulation of epithelial apical–basal polarity. Using live embryo imaging, we show that before the initiation of dorsal transverse folds during Drosophila gastrulation, adherens junctions shift basally in the initiating cells, but maintain their original subapical positioning in the neighbouring cells. Junctional positioning in the dorsal epithelium depends on the polarity proteins Bazooka and Par-1. In particular, the basal shift that occurs in the initiating cells is associated with a progressive decrease in Par-1 levels. We show that uniform reduction of the activity of Bazooka or Par-1 results in uniform apical or lateral positioning of junctions and in each case dorsal fold initiation is abolished. In addition, an increase in the Bazooka/Par-1 ratio causes formation of ectopic dorsal folds. The basal shift of junctions not only alters the apical shape of the initiating cells, but also forces the lateral membrane of the adjacent cells to bend towards the initiating cells, thereby facilitating tissue deformation. Our data thus establish a direct link between modification of epithelial polarity and initiation of epithelial folding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sawyer, J. M. et al. Apical constriction: a cell shape change that can drive morphogenesis. Dev. Biol. 341, 5–19 (2010)
Sweeton, D., Parks, S., Costa, M. & Wieschaus, E. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 112, 775–789 (1991)
Martin, A. C., Kaschube, M. & Wieschaus, E. F. Pulsed contractions of an actin–myosin network drive apical constriction. Nature 457, 495–499 (2009)
Benton, R. & St Johnston, D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115, 691–704 (2003)
Harris, T. J. & Peifer, M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila . J. Cell Biol. 167, 135–147 (2004)
Harris, T. J. & Peifer, M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila . J. Cell Biol. 170, 813–823 (2005)
Krahn, M. P., Buckers, J., Kastrup, L. & Wodarz, A. Formation of a Bazooka–Stardust complex is essential for plasma membrane polarity in epithelia. J. Cell Biol. 190, 751–760 (2010)
McGill, M. A., McKinley, R. F. & Harris, T. J. Independent cadherin–catenin and Bazooka clusters interact to assemble adherens junctions. J. Cell Biol. 185, 787–796 (2009)
Morais-de-Sá, E., Mirouse, V. & St Johnston, D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141, 509–523 (2010)
Walther, R. F. & Pichaud, F. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr. Biol. 20, 1065–1074 (2010)
Lecuit, T. & Lenne, P. F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nature Rev. Mol. Cell Biol. 8, 633–644 (2007)
Martin, A. C., Gelbart, M., Fernandez-Gonzalez, R., Kaschube, M. & Wieschaus, E. F. Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735–749 (2010)
Kametani, Y. & Takeichi, M. Basal-to-apical cadherin flow at cell junctions. Nature Cell Biol. 9, 92–98 (2007)
Cavey, M. & Lecuit, T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 1, a002998 (2009)
Morin, X., Daneman, R., Zavortink, M. & Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila . Proc. Natl Acad. Sci. USA 98, 15050–15055 (2001)
Royou, A., Sullivan, W. & Karess, R. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J. Cell Biol. 158, 127–137 (2002)
Oda, H. & Tsukita, S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J. Cell Sci. 114, 493–501 (2001)
Lighthouse, D. V., Buszczak, M. & Spradling, A. C. New components of the Drosophila fusome suggest it plays novel roles in signaling and transport. Dev. Biol. 317, 59–71 (2008)
Shulman, J. M., Benton, R. & St Johnston, D. The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell 101, 377–388 (2000)
Benton, R. & St Johnston, D. A conserved oligomerization domain in Drosophila Bazooka/PAR-3 is important for apical localization and epithelial polarity. Curr. Biol. 13, 1330–1334 (2003)
Müller, H. A. & Wieschaus, E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila . J. Cell Biol. 134, 149–163 (1996)
McDonald, J. A., Khodyakova, A., Aranjuez, G., Dudley, C. & Montell, D. J. PAR-1 kinase regulates epithelial detachment and directional protrusion of migrating border cells. Curr. Biol. 18, 1659–1667 (2008)
Simões, M. et al. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev. Cell 19, 377–388 (2010)
Marr, D. & Hildreth, E. Theory of edge detection. Proc. R. Soc. Lond. B Biol. Sci. 207, 187–217 (1980)
Maurer, C. R., Jr, Qi, R. & Raghavan, V. A linear time algorithm for computing exact Euclidean distance transforms of binary images in arbitrary dimensions. IEEE Trans. Pattern Anal. Mach. Intell. 25, 265–270 (2003)
Palágyi, K. et al. A sequential 3D thinning algorithm and its medical applications information processing in medical imaging. Lect. Notes Comput. Sci. 2082, 409–415 (2001)
Vincent, L. & Soille, P. Watersheds in digital spaces: an efficient algorithm based on immersion simulations. IEEE Trans. Pattern Anal. Mach. Intell. 13, 583–598 (1991)
Beucher, S. & Meyer, F. In Mathematical Morphology in Image Processing Ch. 12 (ed. Dougherty, E. R. ) 433–481 (Marcel Dekker, 1993)
Lorensen, W. E. & Cline, H. E. Marching cubes: A high resolution 3D surface construction algorithm. SIGGRAPH '87: Proceedings of the 14th annual conference on computer graphics and interactive techniques 21, 163–169 (1987)
Cohen, L. D. & Cohen, I. Finite element methods for active contour models and balloons for 2D and 3D images. IEEE Trans. Pattern Anal. Mach. Intell. 15, 1131–1147 (1991)
Acknowledgements
We thank A. Martin, J. McDonald, D. St Johnston and J. Zallen for providing flies and antibodies; J. Goodhouse and S. Thiberge for assistance in microscopy; G. Deshpande, members of the Wieschaus and Schüpbach laboratories for helpful comments on the manuscript and discussion. This work is supported by a postdoctoral fellowship from the Helen Hay Whitney Foundation to Y.-C.W., a National Institutes of Health/National Institute of General Medical Sciences P50 grant (GM071508) to Z.K. and M.K., and a National Institute of Child Health and Human Development grant (5R37HD15587) to E.F.W. E.F.W. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
Y.-C.W. conceived the project, performed all experiments and analysed the data, except those experiments that involve scanning electron microscopy, which were performed by E.F.W.; Z.K. and M.K. developed the software for three-dimensional reconstruction. Y.-C.W. and E.F.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-13 and Supplementary Movie Legends 1-19. (PDF 1699 kb)
Supplementary Movies
This file contains Supplementary Movies 1-4. (ZIP 12613 kb)
Supplementary Movies
This file contains Supplementary Movies 5-9. (ZIP 10629 kb)
Supplementary Movies
This file contains Supplementary Movies 10-14. (ZIP 21795 kb)
Supplementary Movies
This file contains Supplementary Movies 15-19. (ZIP 24287 kb)
Rights and permissions
About this article
Cite this article
Wang, YC., Khan, Z., Kaschube, M. et al. Differential positioning of adherens junctions is associated with initiation of epithelial folding. Nature 484, 390–393 (2012). https://doi.org/10.1038/nature10938
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10938
This article is cited by
-
Dynamics and functions of E-cadherin complexes in epithelial cell and tissue morphogenesis
Marine Life Science & Technology (2023)
-
Apical–basal polarity and the control of epithelial form and function
Nature Reviews Molecular Cell Biology (2022)
-
β-Catenin nuclear localization positively feeds back on EGF/EGFR-attenuated AJAP1 expression in breast cancer
Journal of Experimental & Clinical Cancer Research (2019)
-
A homeostatic apical microtubule network shortens cells for epithelial folding via a basal polarity shift
Nature Cell Biology (2018)
-
Establishment of the PAR-1 cortical gradient by the aPKC-PRBH circuit
Nature Chemical Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.