Featured
-
-
Article
| Open Access Automated extraction of chemical synthesis actions from experimental procedures
Automated extraction of chemical synthesis actions from experimental procedures
Extracting experimental operations for chemical synthesis from procedures reported in prose is a tedious task. Here the authors develop a deep-learning model based on the transformer architecture to translate experimental procedures from the field of organic chemistry into synthesis actions.
- Alain C. Vaucher
- , Federico Zipoli
- & Teodoro Laino
-
Article
| Open Access Structurally divergent dynamic combinatorial chemistry on racemic mixtures
Structurally divergent dynamic combinatorial chemistry on racemic mixtures
Structurally divergent reactions on racemic mixtures, which produce distinct chemical species from an enantiomeric mixture, are extremely rare in the literature. Here, the authors are able to use a dynamic combinatorial approach to yield structurally divergent, non-isomeric [2]catenanes from an enantiomeric mixture.
- Tiberiu-M. Gianga
- & G. Dan Pantoș
-
Article
| Open Access Asymmetric total synthesis of yuzurimine-type Daphniphyllum alkaloid (+)-caldaphnidine J
Asymmetric total synthesis of yuzurimine-type Daphniphyllum alkaloid (+)-caldaphnidine J
Despite being known for more than 50 years, yuzurimine-type alkaloids have not been accessed by total synthesis. Here, the authors report the first enantioselective total synthesis of (+)-Caldaphnidine J, a highly complex yuzurimine-type Daphniphyllum alkaloid.
- Lian-Dong Guo
- , Yan Zhang
- & Jing Xu
-
Article
| Open Access Heteroatom-bridged molecular belts as containers
Heteroatom-bridged molecular belts as containers
Heteroatom-bearing molecular loops and belts are fascinating but generally difficult to synthesize. Here, the authors demonstrate that O,S-bridged double-stranded molecular belts—cyclophenoxathiins—can be successfully constructed and employed as versatile supramolecular hosts.
- Jialin Xie
- , Xia Li
- & Kelong Zhu
-
Article
| Open Access Upgrading ketone synthesis direct from carboxylic acids and organohalides
Upgrading ketone synthesis direct from carboxylic acids and organohalides
Due to their abundance and importance in organic chemistry, development of methods for ketone synthesis is essential. Here, the authors report a photoredox, nickel and phosphoranyl radical synergistic cross-electrophile coupling of aromatic acids and aryl/alkyl bromides to directly synthesise ketones.
- Rehanguli Ruzi
- , Kai Liu
- & Jin Xie
-
Article
| Open Access A thermally activated and highly miscible dopant for n-type organic thermoelectrics
A thermally activated and highly miscible dopant for n-type organic thermoelectrics
Realizing efficient n-doping in organic thermoelectrics remains a challenge due to dopant-semiconductor immiscibility, poor dopant stability and low doping efficiency. Here, the authors use computer-assisted screening to develop n-dopants for thermoelectric polymers that show record power factors.
- Chi-Yuan Yang
- , Yi-Fan Ding
- & Jian Pei
-
Article
| Open Access Reductive dearomative arylcarboxylation of indoles with CO2 via visible-light photoredox catalysis
Reductive dearomative arylcarboxylation of indoles with CO2 via visible-light photoredox catalysis
Catalytic reductive coupling of two electrophiles and one C = C bond is usually performed by two electron transfer metal catalysis. Herein, the authors show a visible light photoredox-catalyzed successive single electron transfer leading to dearomative arylcarboxylation of indoles with CO2 and generating indoline-3-carboxylic acids.
- Wen-Jun Zhou
- , Zhe-Hao Wang
- & Da-Gang Yu
-
Article
| Open Access Cobalt-catalyzed highly enantioselective hydrogenation of α,β-unsaturated carboxylic acids
Cobalt-catalyzed highly enantioselective hydrogenation of α,β-unsaturated carboxylic acids
A large number of marketed drugs contains a chiral carboxylic acid scaffold. Here, the authors report the asymmetric hydrogenation of α,β-unsaturated carboxylic acids to α-chiral carboxylic acids using a cobalt catalyst bearing an electron-donating chiral diphosphine ligand.
- Xiaoyong Du
- , Ye Xiao
- & Xumu Zhang
-
Article
| Open Access Deazaflavin reductive photocatalysis involves excited semiquinone radicals
Deazaflavin reductive photocatalysis involves excited semiquinone radicals
Flavins and deazaflavins are well suited for photoredox processes but their application in photoreductions is challenging. Here, the authors provide direct evidence of the high reductive power of excited deazaflavin semiquinones and their application in catalytic photodehalogenations.
- Andreas Graml
- , Tomáš Neveselý
- & Burkhard König
-
Article
| Open Access Chemodivergent transformations of amides using gem-diborylalkanes as pro-nucleophiles
Chemodivergent transformations of amides using gem-diborylalkanes as pro-nucleophiles
Amides are versatile synthetic building blocks, however the general stability of the amide bond makes its selective transformation challenging. Here, the authors report a chemodivergent transformation of primary, secondary and tertiary amides by using 1,1-diborylalkanes as pro-nucleophiles.
- Wei Sun
- , Lu Wang
- & Chao Liu
-
Article
| Open Access Asymmetric one-pot transformation of isoflavones to pterocarpans and its application in phytoalexin synthesis
Asymmetric one-pot transformation of isoflavones to pterocarpans and its application in phytoalexin synthesis
Concise total syntheses of 6a-hydroxypterocarpans are sought after due to their broad spectrum of bioactivities. Here, the authors report the asymmetric syntheses of several natural isoflavonoids, including (−)-glyceollin I and (−)-glyceollin II, by means of an asymmetric transfer hydrogenation (ATH) reaction.
- Philipp Ciesielski
- & Peter Metz
-
Article
| Open Access The halogen bond with isocyano carbon reduces isocyanide odor
The halogen bond with isocyano carbon reduces isocyanide odor
Carbon atoms of various species typically function as acceptors of noncovalent interactions when they are part of a π-system. Here, the authors report their discovery of a noncovalent halogen bond involving the isocyano carbon lone pair, which results in adducts with strongly reduced isocyanide odor.
- Alexander S. Mikherdov
- , Alexander S. Novikov
- & Vadim Yu. Kukushkin
-
Article
| Open Access Transition-metal-free formal cross-coupling of aryl methyl sulfoxides and alcohols via nucleophilic activation of C-S bond
Transition-metal-free formal cross-coupling of aryl methyl sulfoxides and alcohols via nucleophilic activation of C-S bond
Cross-coupling processes without the use of transition metals are challenging to achieve. Here, the authors show a transition-metal-free cross-coupling utilizing aryl(heteroaryl) methyl sulfoxides and alcohols to afford alkyl aryl(heteroaryl) ethers and propose a nucleophilic addition mechanism based on experiments and theory.
- Guolin Li
- , Yexenia Nieves-Quinones
- & Tiezheng Jia
-
Article
| Open Access Direct transfer of tri- and di-fluoroethanol units enabled by radical activation of organosilicon reagents
Direct transfer of tri- and di-fluoroethanol units enabled by radical activation of organosilicon reagents
Methods for direct incorporation of tri- and di-fluoroethanol units in molecules are relatively limited. Here, the authors report two organosilicon reagents which are applied to allylation, alkylation and alkenylation reactions as tri- and di-fluoroethanol transfer reagents.
- Xiang Chen
- , Xingxing Gong
- & Xiao Shen
-
Article
| Open Access Site-selective electrooxidation of methylarenes to aromatic acetals
Site-selective electrooxidation of methylarenes to aromatic acetals
Benzylic oxygenation of methylarenes is a direct but challenging method for aldehyde synthesis from simple starting materials. Here, the authors show an electrochemical, site-selective method for the oxidation of methyl benzoheterocycles to aromatic acetals without using chemical oxidants or transition metal catalysts.
- Peng Xiong
- , Huai-Bo Zhao
- & Hai-Chao Xu
-
Article
| Open Access Characterization of glycosyl dioxolenium ions and their role in glycosylation reactions
Characterization of glycosyl dioxolenium ions and their role in glycosylation reactions
Dioxolenium ion intermediates formed from remote positions are hypothesized to direct stereoselective glycosylations. Herein we combine infrared ion spectroscopy, DFT calculations and synthetic work to characterize and study these dioxolenium ions and their role in stereoselective glycosylation reactions.
- Thomas Hansen
- , Hidde Elferink
- & Thomas J. Boltje
-
Article
| Open Access Integrated redox-active reagents for photoinduced regio- and stereoselective fluorocarboborylation
Integrated redox-active reagents for photoinduced regio- and stereoselective fluorocarboborylation
The synthesis of vinylboronates and alkylboronates often suffers from step-tedious and poorly stereoselective procedures. Here, the authors report a bench-stable redox-active reagent for the radical difunctionalization of alkenes and alkynes affording fluorine-containing vinylboronates and alkylboronates.
- Weigang Zhang
- , Zhenlei Zou
- & Yi Pan
-
Article
| Open Access Selective C-H trifluoromethoxylation of (hetero)arenes as limiting reagent
Selective C-H trifluoromethoxylation of (hetero)arenes as limiting reagent
Selective C-H trifluoromethoxylation of pyridines remains a formidable synthetic challenge. Here, the authors report a silver-mediated late-stage C-H trifluoromethoxylation of arenes and heteroarenes as limiting reagents with trifluoromethoxide anion.
- Zhijie Deng
- , Mingxin Zhao
- & Pingping Tang
-
Article
| Open Access A cysteine selenosulfide redox switch for protein chemical synthesis
A cysteine selenosulfide redox switch for protein chemical synthesis
Control of cysteine reactivity is of paramount importance for the synthesis of proteins using native chemical ligation. Here, the authors report a readily cleavable N-selenoethyl group attached to cysteine and apply it to the modular assembly of linear and cyclic polypeptides.
- Vincent Diemer
- , Nathalie Ollivier
- & Oleg Melnyk
-
Article
| Open Access Constructing chiral bicyclo[3.2.1]octanes via palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes
Constructing chiral bicyclo[3.2.1]octanes via palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes
Tandem Heck/carbonylation reaction gives access to ubiquitous carbonyl molecules, however the asymmetric version is rarely studied. Here, the authors synthesize chiral bicyclo[3.2.1]octanes with a palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes with alcohols, phenols and amines.
- Zhenbo Yuan
- , Yuye Zeng
- & Hequan Yao
-
Article
| Open Access Arene dearomatization through a catalytic N-centered radical cascade reaction
Arene dearomatization through a catalytic N-centered radical cascade reaction
Arene dearomatization reactions allow chemists to rapidly build unique chemical architectures from largely available starting materials. Here, the authors show a photocatalytic carboamination/dearomatization cascade process leading to 1,4-cyclohexadiene-fused sultams via N-centered radicals.
- Rory C. McAtee
- , Efrey A. Noten
- & Corey R. J. Stephenson
-
Article
| Open Access Alkyne–Alkene [2 + 2] cycloaddition based on visible light photocatalysis
Alkyne–Alkene [2 + 2] cycloaddition based on visible light photocatalysis
[2 + 2] cycloaddition of alkynes with alkenes would normally require UV light irradiation. Here, the authors report an alkyne–alkene [2 + 2] cycloaddition based on visible light energy transfer photocatalysis, both inter- and intramolecularly, to afford cyclobutenes and 1,3-dienes.
- Sujin Ha
- , Yeji Lee
- & Cheol-Min Park
-
Article
| Open Access Diastereoselective desymmetric 1,2-cis-glycosylation of meso-diols via chirality transfer from a glycosyl donor
Diastereoselective desymmetric 1,2-cis-glycosylation of meso-diols via chirality transfer from a glycosyl donor
Enzyme-catalyzed desymmetric glycosylations are often found in nature, however the corresponding chemical methods are lacking. Here, the authors report a highly diastereoselective desymmetric 1,2-cis-glycosylation of meso-diols found in myo-inositol 1,3,5-orthoesters using a boronic acid catalyst.
- Masamichi Tanaka
- , Koji Sato
- & Kazunobu Toshima
-
Article
| Open Access Room-temperature chemical synthesis of C2
Room-temperature chemical synthesis of C2
Diatomic carbon (C2) is historically an elusive chemical species, considered to require high physical energy for its generation. Here, the authors describe the first room-temperature chemical synthesis of C2 and present experimental evidence for its singlet biradical (quadruple bonding) character and role as a molecular element of nanocarbons.
- Kazunori Miyamoto
- , Shodai Narita
- & Masanobu Uchiyama
-
Article
| Open Access Photoinduced site-selective alkenylation of alkanes and aldehydes with aryl alkenes
Photoinduced site-selective alkenylation of alkanes and aldehydes with aryl alkenes
Dehydrogenative alkenylation of C-H bonds is an atom-economical approach to prepare more complex olefins. Here, the authors use a combination of decatungstate and a cobaloxime catalyst for the photocatalytic dehydrogenative alkenylation of alkanes and aliphatic aldehydes with aryl alkenes.
- Hui Cao
- , Yulong Kuang
- & Jie Wu
-
Article
| Open Access Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones
Synthesis of bridged tricyclo[5.2.1.01,5]decanes via nickel-catalyzed asymmetric domino cyclization of enynones
Tricyclo[5.2.1.01,5]decanes are often found in bioactive natural products; however, their synthesis poses significant challenges. Here, the authors report a nickel-catalyzed asymmetric domino cyclization of enynones, providing a rapid and modular synthesis of bridged tricyclo[5.2.1.01,5]decane skeletons.
- Jiachang Chen
- , Yiming Wang
- & Wangqing Kong
-
Article
| Open Access Synthesis and evaluation of designed PKC modulators for enhanced cancer immunotherapy
Synthesis and evaluation of designed PKC modulators for enhanced cancer immunotherapy
Bryostatin 1 is a unique therapeutic lead, however its scarce natural sources have hampered its use in treatment of human disease. Here, the authors use a scalable synthesis of bryostatin 1 to make close-in analogs which potently induce increased cell surface expression holding potential for immunotherapy.
- Clayton Hardman
- , Stephen Ho
- & Paul A. Wender
-
Article
| Open Access Unusual KIE and dynamics effects in the Fe-catalyzed hetero-Diels-Alder reaction of unactivated aldehydes and dienes
Unusual KIE and dynamics effects in the Fe-catalyzed hetero-Diels-Alder reaction of unactivated aldehydes and dienes
Recently an iron catalyst was developed to catalyze an oxa-Diels-Alder reaction, whose mechanism is unclear yet. Here the authors combine DFT and molecular dynamics simulations with experimental studies to elucidate the unusual iron effect on kinetic isotope effect and dynamics in this reaction.
- Yuhong Yang
- , Xiaoyong Zhang
- & Lung Wa Chung
-
Article
| Open Access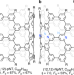 A nitrogen-doped nanotube molecule with atom vacancy defects
A nitrogen-doped nanotube molecule with atom vacancy defects
Replacing carbon atoms in nanocarbons with heteroatoms alters their intrinsic properties, and nitrogen-doped nanocarbons attract much attention in various fields. Here, the authors synthesize a discrete nitrogen-doped nanotube molecule and clarify its structure to reveal unique features of nitrogen dopants.
- Koki Ikemoto
- , Seungmin Yang
- & Hiroyuki Isobe
-
Article
| Open Access Stereoselective gridization and polygridization with centrosymmetric molecular packing
Stereoselective gridization and polygridization with centrosymmetric molecular packing
Gridarenes with well-defined edges and vertices represent versatile nanoscale building blocks for installating frameworks but suffer from lack of stereoselective control during their synthesis. Here, the authors report a diastereoselective gridization of superelectrophilic diazafluorene-containing substrates with crescent shapes into Drawing Hands grids (DHGs).
- Dongqing Lin
- , Ying Wei
- & Wei Huang
-
Article
| Open Access A general 11C-labeling approach enabled by fluoride-mediated desilylation of organosilanes
A general 11C-labeling approach enabled by fluoride-mediated desilylation of organosilanes
Convenient and fast methods to introduce 11C into organic molecules are of great help for molecular imaging studies. Here, the authors developed an efficient incorporation of [11C]CO2 and [11C]CH3I into molecules via a fluoride-mediated desilylation process.
- Wenchao Qu
- , Bao Hu
- & Julie Urgiles
-
Article
| Open Access Enantioselective three-component aminomethylation of α-diazo ketones with alcohols and 1,3,5-triazines
Enantioselective three-component aminomethylation of α-diazo ketones with alcohols and 1,3,5-triazines
Strategies for enantioselective α-aminomethylation of carbonyl compounds rely on the chiral activation of stable ketones substrate. Here, the authors report a rhodium/chiral phosphoric acid cooperative catalysis for the three-component reaction of α-diazo ketones with alcohols and 1,3,5-triazines via imine chiral activation.
- Jiuwei Che
- , Li Niu
- & Wenhao Hu
-
Article
| Open Access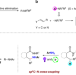 Visible-light promoted regioselective amination and alkylation of remote C(sp3)-H bonds
Visible-light promoted regioselective amination and alkylation of remote C(sp3)-H bonds
C-N bond forming is an established strategy to form amines, which are quintessential in chemical synthesis and in nature. Here, the authors report three classes of photoredox reactions, involving C(sp3)-N coupling between N-centered radicals and alkyl radicals and C(sp3)- C(sp3) coupling via C(sp3)-H alkylation.
- Quanping Guo
- , Qiang Peng
- & Zhaoqing Xu
-
Article
| Open Access Dichloromethylation of enones by carbon nitride photocatalysis
Dichloromethylation of enones by carbon nitride photocatalysis
Long-lived carbon nitride radicals have been used in several photocatalytic reactions. Herein, long-lived potassium poly(heptazine imide) radicals enable synthesis of γ,γ-dichloroketones from enones by addition of CHCl2 moiety, generated from chloroform, to the C=C bond.
- Stefano Mazzanti
- , Bogdan Kurpil
- & Aleksandr Savateev
-
Article
| Open Access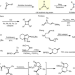 Intermolecular [3+3] ring expansion of aziridines to dehydropiperi-dines through the intermediacy of aziridinium ylides
Intermolecular [3+3] ring expansion of aziridines to dehydropiperi-dines through the intermediacy of aziridinium ylides
Traditional synthesis of stereodefined piperidines requires selective installation of functional groups that can lower efficiency and modularity. Here, the authors assemble stereochemically complex and highly substituted dehydropiperidines via an intermolecular ring expansion between bicyclic aziridines and Rh-supported vinyl carbenes.
- Josephine Eshon
- , Kate A. Nicastri
- & Jennifer M. Schomaker
-
Article
| Open Access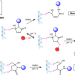 Cysteine-specific protein multi-functionalization and disulfide bridging using 3-bromo-5-methylene pyrrolones
Cysteine-specific protein multi-functionalization and disulfide bridging using 3-bromo-5-methylene pyrrolones
Many reagents have been developed for cysteine-specific protein modification. However, few of them allow for multi-functionalization of a single Cys residue and disulfide bridging bioconjugation. Here the authors report 3-bromo-5-methylene pyrrolones as a simple, robust and versatile class of reagents for cysteine-specific protein modification.
- Yingqian Zhang
- , Chuanlong Zang
- & Chuanzheng Zhou
-
Article
| Open Access Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups
Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups
Aspartimide formation is a frequently occurring problem during peptide synthesis. Here, the authors present cyanosulfurylides as a protection group that masks aspartic acid residues during solid phase peptide synthesis, thus preventing aspartimide formation, and can be removed with N-chlorosuccinimide.
- Kevin Neumann
- , Jakob Farnung
- & Jeffrey W. Bode
-
Article
| Open Access Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids
Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids
Asymmetric activation of amide bonds remains a challenge due to the high stability of amide linkages. Here, the authors show an organocatalytic asymmetric C-N bond cleavage of N-sulfonyl biaryl lactams under mild conditions, to access axially chiral biaryl amino acids.
- Guanjie Wang
- , Qianqian Shi
- & Wei Huang
-
Article
| Open Access Engineering stable radicals using photochromic triggers
Engineering stable radicals using photochromic triggers
Long-standing radical species have raised noteworthy concerns in organic chemistry and but there remains a substantial challenge to produce long-standing radicals by light. Here, the authors demonstrate a stable dithienylethene derived photochromic radical for detection of peroxides and ozone.
- Xuanying Chen
- , Wandong Zhao
- & Liangliang Zhu
-
Article
| Open Access Post-functionalization of dibenzothiophene to functionalized biphenyls via a photoinduced thia-Baeyer-Villiger oxidation
Post-functionalization of dibenzothiophene to functionalized biphenyls via a photoinduced thia-Baeyer-Villiger oxidation
The development of thia-Baeyer-Villiger reactions has been elusive so far due to competitive oxidation of sulfoxides to sulfones. Here, the authors show a thia-Baeyer-Villiger-type oxidations converting dibenzothiophene derivatives into sulfinic esters with t-BuOOH and an iron catalyst under UV irradiation.
- Xiaofeng Ma
- , Yazhou Liu
- & István E. Markó
-
Article
| Open Access A unified approach for divergent synthesis of contiguous stereodiads employing a small boronyl group
A unified approach for divergent synthesis of contiguous stereodiads employing a small boronyl group
Predictable and unified approaches to all possible stereoisomers of acyclic compounds with contiguous stereocentres are rare. Here, the authors disclose a divergent α-functionalization of enolates with either syn or anti selectivity employing a β-boronyl group as a small, directing handle.
- Miao Zhan
- , Zhengwei Ding
- & Pengfei Li
-
Article
| Open Access Ligand-promoted cobalt-catalyzed radical hydroamination of alkenes
Ligand-promoted cobalt-catalyzed radical hydroamination of alkenes
Asymmetric intermolecular hydroamination of alkenes is a challenging process, potentially leading to useful chiral amines. Here, the authors report unsymmetric NNN tridentate ligands promoting the cobalt-catalyzed radical hydroamination of alkenes via hydrogen atom transfer, also in an asymmetric fashion.
- Xuzhong Shen
- , Xu Chen
- & Zhan Lu
-
Article
| Open Access Versatile cobalt-catalyzed regioselective chain-walking double hydroboration of 1,n-dienes to access gem-bis(boryl)alkanes
Versatile cobalt-catalyzed regioselective chain-walking double hydroboration of 1,n-dienes to access gem-bis(boryl)alkanes
Control of regioselectivity in the double hydroboration of dienes to obtain a single organoboron compound is a considerable synthetic challenge. Here, the authors show a cobalt-catalyzed chain-walking double hydroboration of 1,n-dienes to access gem-bis(boryl)alkanes with regioselective control.
- Ming Hu
- & Shaozhong Ge
-
Comment
| Open Access Chemistry glows green with photoredox catalysis
Chemistry glows green with photoredox catalysis
Can organic chemistry mimic nature in efficiency and sustainability? Not yet, but recent developments in photoredox catalysis animated the synthetic chemistry field, providing greener opportunities for industry and academia.
- Giacomo E. M. Crisenza
- & Paolo Melchiorre
-
Article
| Open Access Nucleophilic trifluoromethoxylation of alkyl halides without silver
Nucleophilic trifluoromethoxylation of alkyl halides without silver
Trifluoromethyl ethers are important bioactive targets in pharmaceuticals and agrochemicals, however, their synthesis is often not straightforward. Here, the authors disclose a reagent for the nucleophilic trifluoromethoxylation of alkyl halides without silver and under mild conditions.
- Yan Li
- , Yang Yang
- & Pingping Tang
-
Article
| Open Access Radical-mediated C-C cleavage of unstrained cycloketones and DFT study for unusual regioselectivity
Radical-mediated C-C cleavage of unstrained cycloketones and DFT study for unusual regioselectivity
C-C bond scission of unstrained cycloketones with high regioselectivity is a challenging synthetic task. Here, the authors show a facile C-C cleavage of cyclohexanones and cyclopentanones with unusual selectivity under mild conditions with the aid of an in situ formed side-chain aryl radical.
- Mingyang Wang
- , Man Li
- & Chen Zhu
-
Article
| Open Access Direct C–H difluoromethylation of heterocycles via organic photoredox catalysis
Direct C–H difluoromethylation of heterocycles via organic photoredox catalysis
Heterocycles containing difluoromethyl groups are molecules with potential application in pharmaceutical, agricultural and materials science. Here, the authors show an organophotocatalytic difluoromethylation of heterocycles using O2 as green oxidant and preliminarily study the products’ bioactivity.
- Wei Zhang
- , Xin-Xin Xiang
- & Xin Li
-
Article
| Open Access The combination of asymmetric hydrogenation of olefins and direct reductive amination
The combination of asymmetric hydrogenation of olefins and direct reductive amination
Asymmetric hydrogenation (AH) and direct reductive amination (DRA) are both frequently utilized in chemical industry. Here, the authors combine these two transformations to efficiently convert in one step prochiral olefins into chiral amino compounds.
- Shuai Yuan
- , Guorui Gao
- & Mingxin Chang
-
Article
| Open Access Programmable meroterpene synthesis
Programmable meroterpene synthesis
Meroterpenes are a particularly important class of biologically active bicyclo[3.3.1]nonane-containing molecules. Here, the authors report a general strategy toward these valuable targets based on abiotic annulation/rearrangement steps resulting in a concise total synthesis of garsubellin A.
- Xingyu Shen
- , Chi P. Ting
- & Thomas J. Maimone

 Enantiospecific electrochemical rearrangement for the synthesis of hindered triazolopyridinone derivatives
Enantiospecific electrochemical rearrangement for the synthesis of hindered triazolopyridinone derivatives