« Prev Next »
Eukaryotic flagella and cilia have long been recognized as organelles involved in motility, and their structure and function have both been studied in detail. Almost all motile (secondary) cilia and flagella have the same internal structure and have essentially the same function. Whereas flagella are generally few in number (< 5) and relatively long, cilia are typically short and are present in many copies (> 100) in a cell. The structure of both is based on a "9 + 2" arrangement of microtubules in which there are nine pairs of microtubules around the perimeter, with two single microtubules, which form the central pair. Attached to these microtubules are a variety of other structures, such as the inner and outer arms and the radial spokes (Figure 1). The function of cilia is to move water relative to the cell in a regular movement of the cilia. This process can either result in the cell moving through the water, typical for many single-celled organisms, or in moving water and its contents across the surface of the cell. One example of the latter exists in the epithelial cells that line the human respiratory tract, where cilia constantly move mucus up from the lungs to the back of the throat; another exists in the fallopian tubes, where cilia move the eggs down the tube toward the uterus. Scientists have known for some time that defects in ciliary motility lead to specific disorders in humans, such as sterility in the instance of defective sperm cilia or a condition called situs inversus in which defective embryonic cilia cause internal organs such as the heart to end up on the wrong side of the body.
The Primary Cilium
For a long time, almost all attention was on the motile cilia because their function was readily observable. But scientists, starting with Alexander Kowalevsky had reported the presence of single (nonmotile) cilia in a variety of vertebrate cells (Kowalevsky 1867). These solitary and apparently nonfunctional cilia are far more widespread than the motile type. For example, in humans, only a few cell types have motile cilia, namely sperm, epithelia cells in the bronchi and oviducts, and ependymal cells that line brain vesicles. But virtually all other cells have a primary cilium. Likewise, for many years it was generally assumed that the entire phylum Nematoda, comprising roundworms, completely lacked cilia or flagella. Now it is known that they contain primary cilia, although only in sensory neurons.
What makes primary cilia different from the motile form? First, they lack the central pair of microtubules, which would explain the lack of motility; mutants in other organisms, such as Chlamydomonas, that lack the central pair are generally paralyzed (Adams et al. 1981). Primary cilia also seem to lack dynein, one of the molecular motors needed for motility (Schliwa 2003). In addition, some primary cilia do not project beyond the cell surface, and most, but not all, are very short. What do these organelles do if they are not sticking out of the cell, or motile? Many scientists thought that they might simply be a vestigial organ, with no real role, whereas others thought they might be a place to "park" centrioles when the cell was not dividing. However, even others noted that the primary cilia seemed to appear in key places that involved hearing, sight, and other forms of sensory input. By the 1990s, scientists were starting to look at the primary cilia with increasing interest, but the source of this interest came from an unlikely candidate.
Chlamydomonas and Intraflagellar Transport
At this point, the story takes a strange turn. As is often the case in science, work in an apparently unrelated field provides a key piece of information that changes the way people think about something. The green alga Chlamydomonas has been a popular organism for studying flagella structure and function since the middle of the twentieth century. Over the years, dozens of scientists had used Chlamydomonas to learn a great deal about the structure and function of flagella (Luck 1984; Silflow & Lefebvre 2001), but far less was known about how flagella are assembled. In 1993, the discovery of the intraflagellar transport system (IFT), an intracellular transport system that both builds and maintains cilia and flagella, started to provide answers to this problem (Kozminsky et al. 1993). One offshoot of this discovery of IFT was the key observation that one of the proteins involved in IFT function was the same as one known to be involved in a disease called polycystic kidney disease (PKD) in mice (Pazour et al. 2000). The cells that line the nephron of kidneys have primary cilia, and mice with PKD are unable to assemble cilia properly because of the defective protein. PKD, which is the most prevalent genetic disorder of the kidneys in humans (Wilson 2004), became the first of a series of diseases that are now recognized as being defects in the functioning of primary cilia. The primary cilia (Figure 1).
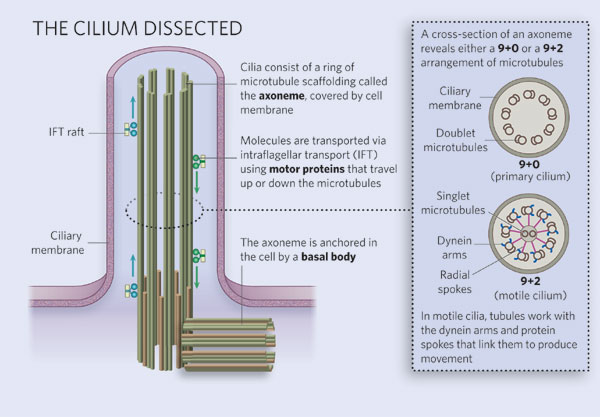
Cilia as Sensory Receptors
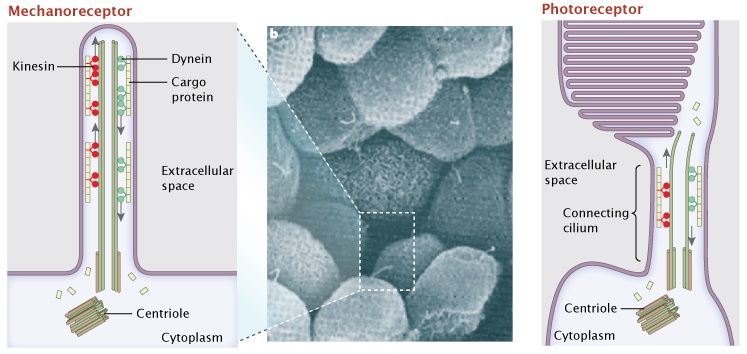
If primary cilia cannot move, what do they do? Defects in primary cilia can cause diseases, which suggests that they have an active role to play, but what is this role? Careful examination of kidney tubule cells showed that the primary cilia bend when exposed to moving liquid (as would be the case in working kidneys). Cells that were exposed to flowing liquid showed a very specific response (Figure 2). The results were interpreted to mean that the normal primary cilia were acting as sensors called mechanoreceptors, responding to flow by opening calcium channels and allowing a rapid influx of calcium ions into the cells.

If primary cilia can act as one type of receptor, can they also act as other types? Yes, they absolutely can. In addition to acting as mechanoreceptors, there are examples of primary cilia being used to detect chemicals, light, osmolarity, temperature, and gravity (Satir, Pedersen, & Christensen 2010). Note that while they may serve as sensory receptor cell specializations, primary cilia can simultaneously support major IFT functions of the cell (Figure 3).
With primary cilia being so prevalent in a variety of receptor functions, it comes as no surprise that they are also involved in signaling. In addition to simple signal transduction, they are now also seen as having an important role in a variety of processes, including development and even some types of memory. Einstein et al. (2010) have shown that the presence of a somatostatin receptor in the primary cilia (SST3) is required for mice to learn to recognize new objects or to recall familiar ones. Furthermore, knockout mice (those lacking both copies of the gene for the receptor) do significantly worse at recognizing and remembering new objects than wild type mice, who have the functional receptor. This response is specific: The mice can still remember such details as the location of objects, but cannot recognize them. The findings were confirmed by treating wild type mice with a SST3 antagonist (a chemical that blocks its action) and observing a similar result. What has changed in these mice? One clue is that the mutant and antagonist-treated mice both showed reduction in cAMP activity in key cell types when compared with the wild type untreated mice. Since cAMP is widely used as an intracellular signaling molecule, those cells unable to make normal amounts of cAMP might have deficiencies in their ability to follow a stimulus.
Ciliopathies
Summary
The primary cilium has gone from being an oddity, with no known function, to an organelle that is at the center of our understanding of both fundamental cell sensory processes and a variety of human disorders. In general, the primary cilium is a sensory organelle that responds to mechanical and chemical stimuli in the environment, communicates that external signal to the cell's interior. However, many questions about primary cilia still remain unanswered. What is their evolutionary origin? Are they the primitive form of cilia, or did they arise from motile cilia? What are the molecular mechanisms behind many of the ciliopathies and some cancers? What components of primary cilia are also essential for motile cilia function and vice versa? What is the role of IFT in both growth of cilia and in their role as sensory organs? Finding the answer to each of these questions will probably require years of research and will most likely create as many more questions as answers.
References and Recommended Reading
Adams, G. M. et al. Central-pair microtubular complex of Chlamydomonas flagella: Polypeptide composition as revealed by analysis of mutants. Journal of Cell Biology 91, 69–76 (1981).
Badano, J. L. et al. The ciliopathies: An emerging class of human genetic disorders. Annual Review of Genomics and Human Genetics 7, 125–148 (2006).
Einstein, E. B. et al. Somatostatin signaling in neuronal cilia is critical for object recognition memory. Journal of Neuroscience 30, 4306–4314 (2010).
Fliegauf, M., Benzing, T., & Omran, H. When cilia go bad: Cilia defects and ciliopathies. Nature Reviews Molecular Cell Biology 8, 880–893 (2007) doi:10.1038/nrm2278.
Hildebrandt, F. & Otto, E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nature Reviews Genetics 6, 928-940 (2005) doi:10.1038/nrg1727.
Jin, H. et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141, 1208–1219 (2010) doi:10.1016/j.cell.2010.05.015.
Kowalevsky, A.. Entwickelungsgeschichte des Amphioxus lanceolatus. Memoires de l'Academie Imperiale des Sciences de St.-Petersbourg VII 11, 1–17 (1867.
Kozminski, K. G., Johnson, K. A., Forscher, P., JL Rosenbaum, J. L. 1993. A Motility in the Eukaryotic Flagellum Unrelated to Flagellar Beating. Proceedings of the National Academy of Sciences 90, 5519–5523 (1993).
Luck, D. J. L. Genetic and biochemical dissection of the eukaryotic flagellum. Journal of Cell Biology 98, 989–994 (1984).
Pazour, G. J., Dickert, B.L., Vucica, Y., Seeley, E. S., Rosenbaum, J. L., Witman, G. B., & Cole, D. G. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene Tg737, are required for assembly of cilia and flagella. Journal of Cell Biology 151, 709–718 (2000).
Marshall, W. F. The cell biological basis of ciliary disease. Journal of Cell Biology 180, 17–21 (2008).
Satir, P., Pedersen, L. B., & Christensen, S. T. The primary cilium at a glance. Journal of Cell Science 123, 499–503 (2010).
Seeley, E. S. & Nachury, M. V. The perennial organelle: assembly and disassembly of the primary cilium. Journal of Cell Science 123, 511–518 (2010).
Schliwa, M. & Woehlke, G. Molecular motors. Nature 422, 759–765 (2003).
Silflow, C. D. & Lefebvre, P. A. Assembly and motility of eukaryotic cilia and flagella. Plant Physiology 127, 1500–1507 (2001).
Wilson, P. D. Polycystic kidney disease. New England Journal of Medicine 350, 151–164 (2004).




 Figure 4: Bardet-Biedl complexes contain a variety of coatlike proteins that can bind and transport various receptor molecules into the cilium.
Figure 4: Bardet-Biedl complexes contain a variety of coatlike proteins that can bind and transport various receptor molecules into the cilium.


























