« Prev Next »
In the 1970s, scientists discovered a novel organelle in apicomplexan parasites, which was named the apicoplast. Like mitochondria and chloroplasts, apicoplasts contain their own DNA. When scientists analyzed apicoplast DNA, they were surprised to learn that apicoplasts shared sequence similarities with plastids (organelles found in the cells of photosynthetic organisms like algae and plants). Could the apicoplast be a vestigial chloroplast? How could apicomplexans and plants be related? What follows is the amazing story of how apicoplasts were discovered and how they have become a promising target for drug development.
Apicomplexans
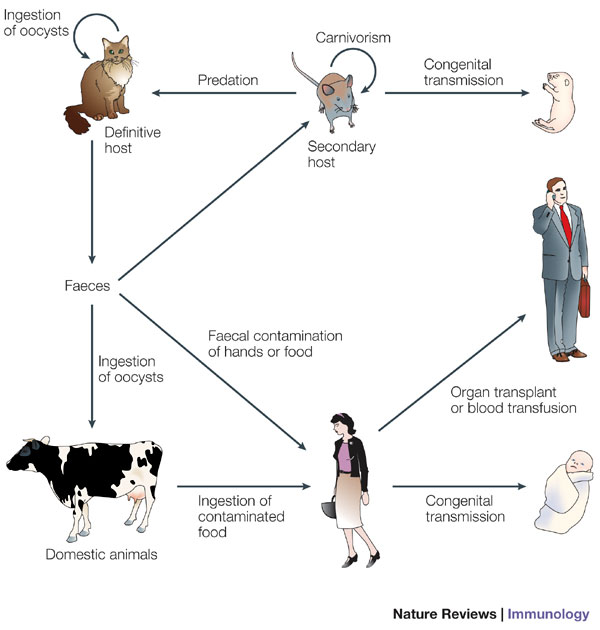
In contrast, human toxoplasmosis produces a mild disease, often without symptoms. It is acquired by ingesting infective Toxoplasma parasites, which can grow in all nucleated cells, including intestinal, muscle and brain cells. The parasite can also be transmitted from mother to fetus. The definitive hosts (in which sexual reproduction occurs) are cats and other felines, and humans are the intermediate hosts simply by accident (Figure 2). The disease is only serious (even fatal) in individuals with weakened immune systems (e.g., AIDS patients), and during pregnancy because it can cause severe developmental malformations. An estimated one third of the world's human population has been infected with Toxoplasma.
Eimeriosis is a disease caused by Eimeria parasites. Unlike Plasmodium and Toxoplasma, Eimeria requires a single host to complete its life cycle. Eimeria infections are a significant cause of disease in poultry, cattle and sheep.
At first glance, these three parasites seem to be very different in terms of their life cycles, hosts, and disease severity. However, despite their differences, the parasites all share a common structure known as the apical complex. This unique structure contains secretory organelles (rhoptries, micronemes, and dense granules), which sequentially secrete enzymes that allow the parasite to invade other cells. Because Plasmodium, Toxoplasma, and Eimeria all have apical complexes, they are members of the same club-the phylum Apicomplexa. All apicomplexans are obligate, intracellular, protozoan parasites, that is, they cannot live or reproduce without a host.
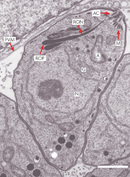
Discovery of the Apicoplast
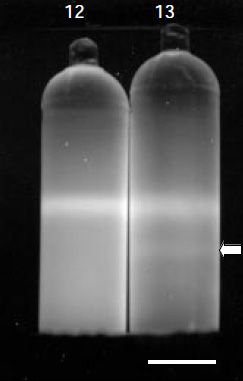
In the1970s, another clue was added to the puzzle by scientists who were characterizing the extrachromosomal DNA molecules in apicomplexans. According to the theory of endosymbiosis, they knew that some cellular organelles, like mitochondria and chloroplasts, contain their own independent genomes, which are separate from the nuclear genome and very similar to bacterial genomes. Using cesium chloride (CsCl) density gradients to separate DNA molecules by centrifugation (Figure 3), the scientists isolated what they believed to be mitochondrial DNAs from P. knowlesi (primate malaria parasites) and P. berghei (rodent malaria parasites). Araxie Kilejian, a post-doctoral scientist at Rockefeller University was intrigued by the results. She wondered why mitochondrial DNAs had not been detected in P. lophurae and P. gallinaceum, since both avian malaria parasites contained visible mitochondria. Kilejian also wanted to learn more about the nature of these extrachromosomal molecules. She soon realized that P. lophurae's extrachromosomal DNAs had the same density as the nuclear DNA, making it difficult to distinguish between them using regular CsCl gradients. She decided to perform careful sub-cellular fractionations and electron micrograph examinations. Using these methods, she succeeded in isolating a population of circular molecules that were estimated to be 10.3 µm (micrometers) in length and 27–kilobases in size (Kilejian 1975). A kilobase, or kb, is the unit of measure used by molecular biologists to measure the size of DNA or RNA fragments. One kb contains 1000 nucleotide base pairs.
At the time, the prevailing "rule of the ring" dogma was that most, if not all, mitochondrial DNAs were circular, so Kilejian mistook these circular structures for mitochondrial DNAs. Later, several groups confirmed her discovery by isolating equivalent extrachromosomal, circular DNA molecules from other apicomplexan species, including P. berghei (11.3 µm), T. gondii (13 µm), P. knowlesi (11.6 µm), and the human malarial parasite, P. falciparum (11.1 µm). Since the experimental data available did not suggest any alternative interpretation, scientists continued to believe that the circular, extrachromosomal DNAs were mitochondrial DNA until late in the 1980s.
In 1987, the acceptance of the "rule of the ring" dogma was called into question when a second extrachromosomal genome — a tandemly arranged, 6-kb, linear DNA molecule — was identified in the rodent malarial parasite P. yoelli (Vaidya & Arasu 1987, Vaidya et al. 1989). Analysis of its DNA and protein sequences showed that it had similarities to mitochondrion-encoded proteins. Two years later, Iain Wilson and his colleagues identified a similar 6-kb element in P. falciparum, and to everyone's surprise, they confirmed that the 6-kb element was the real mitochondrial DNA.
How did they prove that the 6-kb element was the mitochondrial genome? Wilson and his colleagues sequenced the element and detected typical mitochondrial genes in the genome, such as the cytochrome and cytochrome oxidase genes (Feagin et al. 1991). Later, they demonstrated that when purifying subcellular fractions enriched in mitochondria, only the linear genome co-purified with the mitochondria, not the circular DNA. This confirmed that the mitochondria and circular DNA were located in different cellular compartments (Wilson et al. 1992). The scientists could not have been more intrigued. Since the 6-kb element represented the mitochondrial genome, what was the nature of the circular extrachromosomal DNA element? The scientists began exploring alternative explanations for the origin and function of the circular genome.
An Organelle with a Green Past
Iain Wilson teamed with an expert mitochondriologist, Donald Williamson, and researchers Malcolm Gardner and Jean Feagin to study malarial extrachromosomal DNAs. In 1991, the group published a paper in Parasitology Today (now Trends in Parasitology) entitled "Have malaria parasites three genomes?" (Wilson et al. 1991). This title was intentionally provocative. At the time, biologists agreed - and this was the second dogma - that the only eukaryotic cells with three different genomes were the plants and algae, which had nuclear, mitochondrial and plastid genomes. In the Parasitology Today article, Wilson and his group analyzed the circular extrachromosomal DNA. They determined that the size of the molecule was 35 kb using restriction mapping and sequencing (Gardner et al. 1991a). The scientists learned that the circular element contained large inverted repeats with duplicated sets of ribosomal RNAs (rRNAs), which was characteristic of chloroplast genomes. Moreover, when they performed a phylogenetic analysis of the conserved regions of the small subunit rRNA, they noticed similarities between the extrachromosomal genome and several prokaryotic and chloroplast counterparts (Gardner et al. 1991b). Wilson and his colleagues' results were leading them to the conclusion that malaria contained a plastid. Where was this DNA molecule located?
Many scientists participated in the race to locate the 35-kb DNA molecule within the parasite's cell. Araxie Kilejian suggested the possibility that the 35-kb element resided in the "spherical bodies," but there was no experimental evidence to support her hypothesis (Kilejian 1991). It would take another six years before the 35-kb DNA was conclusively shown to associate with the "spherical bodies," following cross-field collaborations between plant scientists and parasitologists. How did these collaborations come about?
A Definition of a New Organelle
One year later, in 1997, the McFadden group's results were confirmed by Sabine Köhler and her colleagues (one of whom was Iain Wilson). The general conclusion was that the apicoplast was indeed a vestigial plastid — a finding that broke another dogma. In their article, Köhler and colleagues proposed naming the recently identified organelle the "apicoplast," which was a combination of the words "apicomplexan" and "plastid" (Köhler et al. 1997). Finally, the new organelle had a name. Since then, apicoplasts have been detected in all apicomplexan parasites studied, with the exception of Cryptosporidium (responsible for human cryptosporidiosis) (Figure 6).
The analysis of both the genomic sequence and the organization of the 35-kb apicoplast genome led to two main conclusions. The first was that the apicoplast genome encodes enough transfer RNAs (tRNAs) and ribosomal genes for a minimal, but sufficient, translation of the protein-encoding genes present in the element. The second conclusion was that the genomic organization showed a close relationship to algae (Wilson et al. 1996). In 2008, in an exciting twist in the story, scientists studied a minute marine alga that provided evidence that apicomplexan parasites evolved from photosynthetic algal ancestors (Keeling 2008).
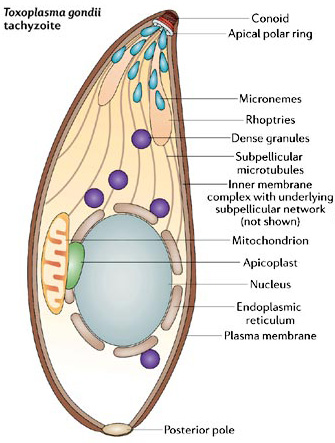
The Apicoplast as a Drug Target
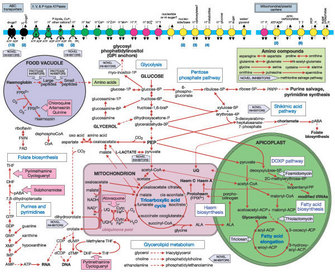
Two different lines of evidence showed that apicoplasts are essential in the apicomplexan parasites. First, chemicals affecting apicoplast metabolism resulted in parasite death. Second, parasites that are unable to replicate the apicoplast also die. Amazingly, in both cases, the parasites only die in the next generation. This means that the parasites can survive with no apicoplast (or with a chemically damaged apicoplast) while remaining in the infected host cell. However, the parasite is unable to establish a successful new infection. This phenomenon is called "delayed death". How is this possible? One hypothesis is that the apicoplast synthesizes a molecule that is needed for the infection process (Ralph et al. 2004).
Since apicoplasts share evolutionary similarities with chloroplasts and prokaryotes (cyanobacteria), they stand out as an attractive target for known antibiotics and herbicides. Today, researchers have learned that apicoplasts are sensitive to several antibacterials and herbicides. Plant scientists are already exploring non-toxic herbicides that may act upon the apicoplast, and together with parasitologists, they are using bioinformatics and experimental approaches to identify key proteins and metabolic pathways in order to develop new drugs (Ralph et al. 2004) (Figure 7).
Summary
Apicoplasts, with their green past, present a unique difference between the host and the parasite, offering an excellent target for the development of new chemotherapeutic alternatives. Could apicoplasts be the long searched for "Achilles' heel" to prevent the spread of malaria? Only time will tell. What is certain is that it will no longer be uncommon to see plant scientists reading journals on parasitology, or parasitologists making their way through plant journals.
References and Recommended Reading
Egea, N. & Lang-Unnasch, N. Phylogeny of the large extrachromosomal DNA of organisms in the phylum Apicomplexa. Journal of Eukaryotic Microbiology 42, 679–684 (1995). doi: 10.1111/j.1550-7408.1995.tb01615.x
Feagin, J. E., Gardner, M. J., et al. The putative mitochondrial genome of Plasmodium falciparum. Journal of Eukaryotic Microbiology38, 243–245 (1991). doi: 10.1111/j.1550-7408.1991.tb04436.x
Gardner, M. J., Feagin, J. E., et al. Organization and expression of small subunit ribosomal RNA genes encoded by a 35–kilobase circular DNA in Plasmodium falciparum. Molecular and Biochemical Parasitology 48, 77–88 (1991b). doi:10.1016/0166-6851(91)90166–4
Gardner, M. J., Williamson, D. H., et al. A circular DNA in malaria parasites encodes an RNA polymerase like that of prokaryotes and chloroplasts. Molecular and Biochemical Parasitology 44, 115–23 (1991a). doi:10.1016/0166-6851(91)90227–W
Keeling, P. J. Bridge over troublesome plastids. Nature 451, 896–897 (2008). doi:10.1038/451896a
Kilejan, A. Circular mitochondrial DNA from the avian malarial parasite Plasmodium lophurae. Biochimica et Biophysica Acta 390, 276–284 (1975). doi:10.1016/0005-2787(75)90348–2
Kilejian, A. Spherical bodies. Parasitology Today 7, 309; author reply 309 (1991).
Köhler, S., Delwiche, C. F., et al. A plastid of probable green algal origin in apicomplexan parasites. Science 275, 1485–1488 (1997). doi: 10.1126/science.275.5305.1485
McFadden, G. I., Reith, M. E., et al. Plastid in human parasites. Nature 381, 482 (1996). doi:10.1038/381482a0
Ralph, S. A., van Dooren, G. G., et al. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nature Reviews Microbiology 2, 203–16 (2004). doi:10.1038/nrmicro843
Scholtyseck, E. & Piekarski, G. Electron microscopic studies on merozoites of Eimeria (Eimeria perforans and E. stidae) and Toxoplasma gondii. On the systematic position of T. gondii. Zeitschrift für Parasitenkunde 26, 91–115 (1965).
Vaidya, A. B. & Arasu, P. Tandemly arranged gene clusters of malaria parasites that are highly conserved and transcribed. Molecular and Biochemical Parasitology 22, 249–257 (1987). doi:10.1016/0166-6851(87)90056–9
Vaidya, A. B., Akella, R., et al. Sequences similar to genes for two mitochondrial proteins and portions of ribosomal RNA in tandemly arrayed 6-kilobase-pair DNA of a malarial parasite. Molecular and Biochemical Parasitology 35, 97–107 (1989). doi:10.1016/0166-6851(89)90112–6
Wilson, R. J. M., Fry, M., et al. Subcellular fractionation of the two organelle DNAs of malaria parasites. Current Genetics 21, 405–408 (1992). doi: 10.1007/BF00351702
Wilson, R. J. M., Gardner, M. J., et al. Have malaria parasites three genomes? Parasitology Today 7, 134–136 (1991). doi:10.1016/0169–4758(91)90276–T
Wilson, R. J. M., Denny, P. W., et al. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. Journal of Molecular Biology 261, 155–172 (1996). doi:10.1006/jmbi.1996.0449



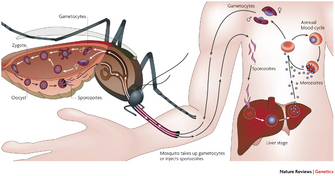
 Figure 1: Plasmodium falciparum life cycle
Figure 1: Plasmodium falciparum life cycle