« Prev Next »
The ability of cells to interact with one another and with their environment relies on a large number of proteins that need to be in the right proportion and in the right location. For this reason, eukaryotic cells have evolved complex sorting machineries to ensure the dynamic and strictly controlled flow of proteins between cellular compartments. This machinery exists in both plants and animals, and it involves vesicular structures called endosomes.
What Are Endosomes?
Endosomes are primarily intracellular sorting organelles. They regulate trafficking of proteins and lipids among other subcellular compartments of the secretory and endocytic pathway, specifically the plasma membrane Golgi, trans-Golgi network (TGN), and vacuoles/lysosomes. Endosomes receive cargo (proteins and lipids) from both the biosynthetic and the endocytic pathways. For instance, proteins that are destined for the vacuoles/lysosomes are synthesized at the endoplasmic reticulum, trafficked through the Golgi, sorted at the TGN, and then sent to endosomes for their final vacuolar/lysosomal delivery (Figure 1).
Endosomes also receive plasma membrane proteins that are internalized by endocytosis (Figure 1). At endosomes, these proteins are either recycled back to the plasma membrane or are sorted for degradation. The recycling of plasma membrane proteins mostly occurs at early and recycling endosomes; their degradative sorting is achieved in intermediate/late endosomes, which are also called multivesicular bodies (MVBs) or multivesicular endosomes.

Endosomes and the Plasma Membrane
At the plasma membrane, several types of proteins and protein complexes mediate cell-to-cell communication and the exchange of substances with the cell's environment. For example, plasma membrane receptors bind ligand molecules (such as hormones) and trigger downstream reaction within the cell. In addition, plasma membrane ion channels allow the release and incorporation of ions that act in signaling cascades. Therefore, when, where, and how many of these proteins are indeed located at the plasma membrane affects the responses of the cell.
How is plasma membrane protein composition controlled? There are several control mechanisms, most of which depend on endosomal trafficking pathways. Plasma membrane proteins reach the endosomes by endocytosis and then are either recycled, degraded, or participate in a signaling pathway. Although endosomal systems are heavily characterized in animal cells, plant cells are less well understood. What follows is a discussion of the endosomal system in plants, with a comparison to parallel mechanisms in animals.
Protein Recycling via Endosomes
Some proteins in the plant plasma membrane are constantly cycled in and out of the membrane. These proteins are exchanged between the membrane and the "early" endosomes. Examples of plant proteins that go through this cycling are the auxin carrier, PIN-FORMED1 (PIN1); the water channel, PIP2; and the K+ channel, KAT1. This constant exchange of proteins between the plasma membrane and early endosomes is called constitutive cycling. This type of cycling allows cells to control rapid changes in plasma membrane composition by having a pool of plasma membrane proteins available in early nearby endosomes.
In mammalian cells, constitutive cycling is tightly regulated by hormones such as insulin or vasopressin, which can change the relative rates of endocytosis and control the concentration of plasma membrane proteins, including channels and receptors. Similarly, plant hormones also affect endocytosis and endosomal constitutive cycling. The plant hormone auxin, which coordinates many growth and developmental processes in plants, has a general inhibitory effect on endocytosis (Paciorek et al. 2005). In contrast, another plant hormone, absicic acid, stimulates endocytosis, specifically the internalization and recycling of KAT1 channel important in the regulation of stomatal opening (Sutter et al. 2007).
How much do we know about the molecular machinery that controls the recycling of plasma membrane proteins in plants? Gerd Jurgen and coworkers identified one of the first endosomal recycling components when they screened for abnormally shaped embryos/seedlings, a protein called GNOM (means "gnome" in German). GNOM is a plant-specific protein that facilitates the exchange of GDP/GTP in ADP-ribosylation factor (ARF) GTPases (enzymes that are very important for the formation of trafficking vesicles). It mediates the constitutive recycling of the auxin carrier PIN1 from endosomal compartments to the plasma membrane.
Many PIN proteins show polarized localization; that is, they are either in the basal or the apical part of the cell. The polarized localization complements PIN1 function in polarized auxin transport. GNOM is involved in the endosomal recycling of PIN1 to the basal plasma membrane in provascular cells. By analyzing mutants with defective GNOM proteins, researchers have found that the endosomal recycling of PIN1 mediated by GNOM is critical for the polarized localization of PIN1.
Plants harboring strong GNOM mutant alleles are not able to properly regulate either the polarized localization of PIN1 or polar auxin transport and therefore develop only rudimentary embryonic organs (Steinmann et al. 1999; Geldner et al. 2001). However, GNOM does not affect the recycling of all plasma membrane proteins. For example, the auxin influx carrier Auxin-resistant (AUX1) seems to be recycled from endosomes to the plasma membrane in a GNOM-independent manner (Kleine-Vehn et al. 2006),which implies that the trafficking of auxin carrier proteins — and therefore the regulation of auxin transport — depends on multiple endosomal trafficking mechanisms.
Another endosomal complex called the retromer is important for the recycling of receptors that mediate the transport of proteins from the TGN to the vacuole (Niemes et al. 2010). In plants, mutations in retromer subunits seem to also affect the localization of some plasma membrane auxin carriers (Jaillais et al. 2006). However, researchers are still analyzing the exact mechanisms by which the retromer may control the localization of plasma membrane proteins in plants.
Signaling via Endosomes
Many receptors, such as receptor tyrosine kinases and G-protein-coupled receptors, are activated at the plasma membrane. However, these activated receptors also signal from endosome membranes, where they can physically interact with some of their downstream signaling factors. In animals, researchers first realized the importance of endosomes as signaling platforms when performing subcellular fractionation and immunoprecipitation experiments. They noticed that receptors such as the epidermal growth factor receptor and insulin receptors, after activation with their corresponding ligands, accumulated in endosomes. In plants, endocytosis and endosomal trafficking are important for signaling via several plasma membrane kinase receptors, such as steroidal plant hormone receptor Brassinosteroid Insensitive 1 (BRI1), which controls cell expansion and division (Geldner et al. 2007).
How Do Endosomes Degrade Membrane Proteins?
Degrading a membrane protein is not trivial. Soluble proteins that are not anchored into membranes can be degraded by cytoplasmic proteases that belong to the 26S proteosome degradation pathway. But how does a cell degrade a protein that is inserted into a membrane? Most plasma membrane proteins are tagged for degradation by special enzymes called E3 ligases. These ligases attach an ubiquitin molecule to specific lysine residues on the target protein, which identifies the protein as ready for degradation. In many organisms, ubiquitination is enough to trigger internalization of plasma membrane proteins and their subsequent delivery to endosomes (Figure 1). Endosomal protein complexes then recognize ubiquitinated membrane proteins and sort them into vesicles inside the endosomal lumen, giving rise to multivesicular endosomes or MVBs. The endosomal invagination process is quite unique. Unlike most vesiculation processes in which membrane buds protrude into the cytoplasm, the endosomal vesiculating membrane buds away from the cytoplasm or toward the inside of the endosome. As a result, the vesicle forms inside the endosome. When the MVBs fuse with vacuoles/lysosomes, which are hydrolytic compartments, the endosomal vesicles are released in the vacuolar lumen and are degraded (see Figure 1).

Bit by bit, by meticulous experimentation in tissue culture as well as in live animals, researchers have discovered how the different ESCRT complexes form MVBs. ESCRT-0 recognizes, selects, and clusters the ubiquitinated membrane proteins on the endosomal membrane. ESCRT-I and ESCRT-II induce membrane deformation at the neck of the nascent vesicle. And ESCRT-III, together with an ATPase called Vps4p/SKD1, is important for membrane fission and the release of the nascent vesicle into the MVB lumen (Figure 2). Vps4p/SKD1 also participates in the disassembly and recycling of the ESCRT coat from the endosomal membrane, and, notably, its ATPase activity provides the main energy source required to form MVB vesicles.
Although ubiquitination is the predominant mechanism for tagging proteins slated for sorting by the ESCRT machinery, there are numerous examples in which it may not be required for MVB sorting. In these cases, the recognition of the MVB cargo proteins is mediated by sorting motifs (short sequences in the protein) located in the cytoplasmic, transmembrane, or luminal regions of cargo proteins.
Is There Anything Special About Plant Endosomes?
Indeed, there are some special things about plant endosomes. First, not all ESCRT proteins in animals are necessary in plants. For instance, no ESCRT-0 subunits have been identified in plants that enable the first step of MVB vesicle formation (Leung, Dacks & Field 2008). Whereas animals cannot survive without a functional ESCRT-0 complex, plants and all other eukaryotic groups (except animals and fungi) never evolved an ESCRT-0 complex but still can successfully perform ESCRT-based endosomal sorting functions. A pending question is: How do the initial recognition steps in MVB formation occur in plants in the absence of the ESCRT-0 complex?
A second noteworthy difference of plant endosomes is their structural and functional organization. Whereas MVBs are commonly observed in plant cells, other types of endosomes, such as the typical tubulo-vesicular early endosomes found in mammals, are not found in plants. Instead, researchers have found that the TGN itself or a TGN-derived compartment in plants acts as an early endosome, receiving recently endocytosed material from the plasma membrane (Dettmer et al. 2006; Lam et al. 2007; Viotti et al. 2010). Do MVBs derive directly from modified TGN compartments? Do they arise from the fusion of TGN-derived and endocytic vesicles? Researchers are still actively researching these questions.
Similar to mammals, plant MVBs also have a fundamental role in the delivery of cargo to vacuoles. Plant cells rely on vacuoles for multiple cellular functions, such as maintenance of turgor pressure, ion homeostasis, sequestration of toxic compounds, and protein and mineral storage. As a method of delivering materials to vacuoles, MVBs bring newly synthesized soluble and membrane-anchored proteins coming from the Golgi/TGN. However, MVBs do not act only as passive carriers.
Some soluble proteins en route to the vacuole are proteolytically processed inside MVBs. Scientists learned about this mechanism by examining seed storage protein propeptides (small peptides that are removed from a protein precursor during the proteolytic processing) inside MVBs (Otegui et al. 2006). These propeptides are very abundant in early stages of MVB formation but not in mature MVBs, indicating that there are active proteases inside plant MVBs and illustrating yet another difference compared with animal cells.
How Are ESCRT Protein Functions Different in Plant Cells?
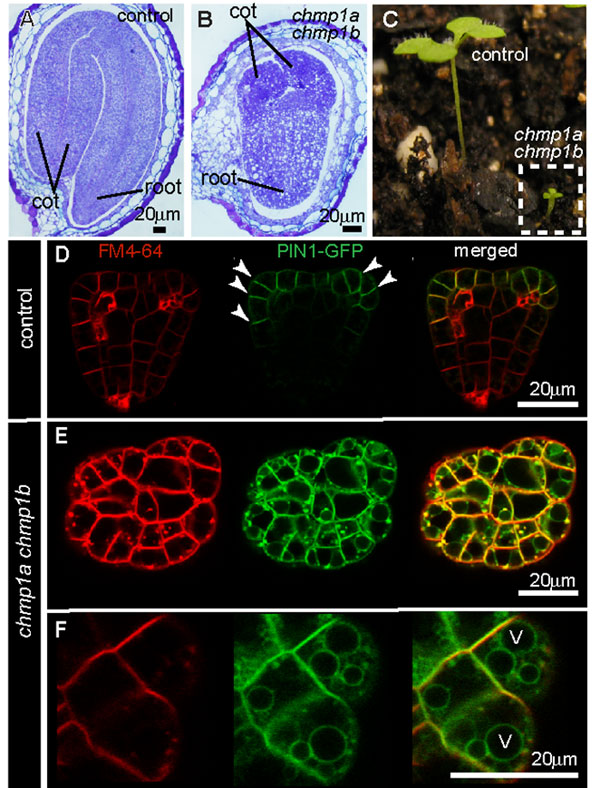
Plants contain genes that encode the main components of the ESCRT machinery, with the exception of ESCRT-0. However, we only know the endosomal functions of a few of these proteins in plants, such as the ESCRT-I component ELCH and the ESCRT-III-related proteins SKD1, LIP5 and CHMP1. Researchers have studied the endosomal functions of these proteins mostly in the model plant, Arabidopsis thaliana, and in corn. Although all ESCRT and ESCRT-related proteins are involved in MVB sorting at the cellular level, mutations in different components can lead to different phenotypes at the whole plant level. To understand the different functions of these genes, researchers use these phenotypes to discern the effects of experimental genetic manipulations and combine this knowledge with what we know about parallel proteins in animal cells (Figure 3).
Summary
Despite being small and structurally simple organelles, endosomes perform an amazing range of tightly controlled sorting events that affect a multitude of signaling processes in cells. In fact, endocytosis and endosomal trafficking are of paramount importance in key plant processes such as embryo differentiation, gravitropism (responses of roots and shoots to gravity), guard cell movement during stomata opening, cell wall remodeling, the regulation of hormone auxin and ion transport, self-incompatibility responses during pollen tube growth, and defense responses against pathogens. Researchers are only beginning to understand how the coordinated actions of protein complexes associated with endosomes control some of these sorting events.
References and Recommended Reading
Azmi, I. et al. Recycling of ESCRTs by the AAA-ATPase Vps4 is regulated by a conserved VSL region in Vta1. Journal of Cell Biology 172, 705–717 (2006).
Dettmer, J. et al. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18, 715–730 (2006).
Geldner, N. et al. 2 Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413, 425–428 (2001).
Geldner, N. et al. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Development 21, 1598–1602 (2007).
Haas, T. J. et al. The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYST-INTERACTING PROTEIN5. Plant Cell 19, 1295–1312 (2007).
Hurley, J. H. & Hanson, P. I. Membrane budding and scission by the ESCRT machinery: It's all in the neck. Nature Reviews Molecular Cell Biology 11, 556–566 (2010).
Kleine-Vehn, J. et al. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18, 3171–3181 (2006).
Jaillais Y et al. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature 443, 106–109 (2006).
Lam, S. K. et al. Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19, 296–319 (2007).
Leung, K. F., Dacks, J. B. & Field, M. C. Evolution of the multivesicular body ESCRT machinery; retention across the eukaryotic lineage. Traffic 9, 1698–1716 (2008).
Nickerson, D. P., West, M. & Odorizzi, G. Did2 coordinates Vps4-mediated dissociation of ESCRT-III from endosomes. Journal of Cell Biology 175, 715–720 (2006).
Niemes, S. et al. Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant Journal 61, 107–121 (2010).
Otegui, M. S. et al. The proteolytic processing of seed storage proteins in Arabidopsis embryo cells starts in the multivesicular bodies. Plant Cell 18, 2567–2581 (2006).
Paciorek, T. et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256 (2005).
Spitzer, C. et al. The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development 133, 4679–4689 (2009).
Steinmann, T. et al. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286, 316–318 (1999).
Sutter, J. U. et al. Abscisic acid triggers the endocytosis of the arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Current Biology 17, 1396–1402 (2007).
Teis, D., Saksena, S. & Emr, S. D. SnapShot: The ESCRT machinery. Cell 137, 182–182.e1 (2009).
Viotti, C. et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22, 1344–1357 (2010).
Winter, V. & Hauser, M-T. Exploring the ESCRTing machinery in eukaryotes. Trends in Plant Science 11, 115–123 (2006).
Wollert, T. & Hurley, J. H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 464, 864-869 (2010) doi:10.1038/nature08849.



 Figure 2: Simplified mode of action of ESCRT and ESCRT-related components during endosomal vesiculation
Figure 2: Simplified mode of action of ESCRT and ESCRT-related components during endosomal vesiculation


























