« Prev Next »
Many adult-onset genetic disorders are progressive (meaning that they get worse over time) and have long-term health consequences. Thus, when a person is at risk for such a disorder, he or she may consider undergoing genetic testing. When the consequences of the specific disorder in question are treatable, most people would agree that genetic testing makes sense. But what about disorders for which no preventative measures or treatments are available? What happens, for instance, when a young adult is found to have the mutation that causes a fatal disorder? Would such a person still be able to buy insurance or get the job promotion he or she applied for? How would this information affect his or her family and social life? As these questions illustrate, genetic testing for potentially lethal disorders is an area fraught with ethical, legal, and social concerns.
Understanding Huntington’s Disease
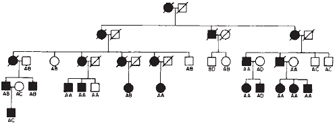
One devastating genetic disorder that exemplifies these difficult ethical and social concerns is Huntington's disease (HD). Symptoms of HD, which include uncontrolled movements, loss of mental abilities, and emotional disturbances, are caused by the progressive death of neurons in certain areas of the brain. Death from related complications usually occurs 10 to 30 years following onset of symptoms. There is currently no treatment for this disease, nor is there anything an affected person can do to prevent the inevitable onset of symptoms.
HD is an autosomal dominant disorder with complete penetrance; therefore, any child of a person with HD has a 50% chance of developing the disease. The genetic basis of Huntington's, discovered in 1992, is an expansion of a trinucleotide repeat within the coding region of the huntingtin gene (La Spada et al., 1992; Huntington's Disease Collaborative Research Group, 1993). Normally, this gene contains between 7 and 35 CAG repeats. In a person with HD, however, the gene contains more than 35 repeats. The age of onset of symptoms is inversely related to the number of repeats; generally, the more repeats that are present, the earlier the age of onset. A person with 40 CAG repeats can expect the onset of symptoms to occur anywhere from 40 to 70 years of age.
HD presents many unique challenges to affected families. Given the age of onset of symptoms, many individuals have already passed the disease to their children before their own diagnosis (Figure 1). Also, many people with HD have had the experience of seeing a parent, grandparent, or other family member suffer with the condition, due to the high penetrance and founder effect (meaning that de novo mutations are very rare) of HD. Genetic testing is available to those at risk for the disease and can indicate with certainty whether an individual is affected, but many people opt not to be tested. In fact, a 13-year Canadian study revealed that only 3% to 24% of at-risk individuals get predictive testing (Creighton et al., 2003). This percentage is surprisingly low, especially given the fact that surveys taken shortly after predictive testing for HD became available indicated that between 66% and 79% of individuals at risk for the disorder would seek genetic testing to determine their status (Mastromauro et al., 1987; Kessler et al., 1987). What, then, might explain this discrepancy?
Reproductive and Other Familial Concerns
The decision to undergo genetic testing for an inherited disease is an intensely personal choice, influenced by the perceived treatability and preventability of the disorder, as well as various reproductive implications. In the case of HD, although there is no way to treat or prevent this condition, knowing one's status could influence many life decisions. For example, if you learned you had inherited the HD mutation, would you decide to have children of your own and risk passing the gene on to your children? Perhaps you would consider using assisted reproductive technology (ART), such as preimplantation genetic diagnosis, to make sure only unaffected embryos were allowed to develop to term. But what if you already had children? Would you still get tested? What if test results indicated that you were likely to begin showing symptoms at age 40? How might this affect the way you live your life? Conversely, what if testing revealed that you had not inherited the mutation, but that your siblings and their children had? Feelings of guilt and depression are not uncommon in these situations. Finally, what if you chose not to get tested at all? Research has shown that individuals with a family history of genetic disorders who know they are at risk but choose not to get tested can have difficulties in social interactions (McConkie-Rosell et al., 2008). Each scenario is complex, especially given the incurable nature of HD.
The decision to undergo genetic testing can only be made by the individual at risk for a disorder. Once a test has been conducted and the results are known, however, a new, family-related ethical dilemma is born: Should a carrier of a known genetic risk be obligated to tell his or her relatives (Forrest et al., 2007; Gaff et al., 2007)? This very question has begun to challenge the well-established medicolegal principles of confidentiality and privacy. Although some people feel that an individual who is found to carry a dominant gene for Huntington's disease has an ethical obligation to disclose that fact to his or her siblings, there currently is no legal requirement to do so. In fact, requiring someone to communicate his or her own genetic risk to family members who are therefore also at risk is considered by many to be ethically dubious.
So, then, should a medical professional get involved if he or she becomes aware that communication within a family has failed or is blocked? A professional's actions in such situations depend in part upon the nature of the information available (e.g., do the test results only estimate risk levels, or are they definitive?), as well as the implications of the condition. For instance, a greater imperative to breach confidentiality may be felt when preventative treatment options are available or when reproductive choices are at stake.
Genetic Testing and Privacy Concerns
Another important consideration when deciding whether to undergo genetic testing is the possibility that if someone knows you are likely to develop a genetic disorder in the future, he or she could use that information against you. For example, if an employer knows that an employee is likely to be diagnosed with cancer or HD, the employer might not want to retain that employee. Similarly, a person who is known to have a high risk for a genetic condition may have difficulty obtaining insurance because he or she is likely to run up medical bills that would be costly to the insurance company. Because we cannot control our genes, it is unfair to discriminate against a person's genetic predispositions. In the United States, the Health Insurance Portability and Accountability Act of 1996 (HIPAA) was the first federal law to provide protections against genetic discrimination, but its intentions fell short. Specifically, under HIPAA, insurance companies were not prevented from charging higher rates to customers based on genetic information, nor were they prevented from collecting genetic data or requiring applicants to undergo genetic testing. Thus, a more comprehensive federal measure, the Genetic Information Nondiscrimination Act (GINA), was signed into law in May 2008 (Allison, 2008). GINA, spearheaded by members of Congress like Representative Louise Slaughter, GINA took ten years to pass. Ideally, now that the legislation is law, the protections provided by this act will foster an atmosphere in which Americans feel safe to take advantage of appropriate genetic testing, as well as to share test results with any other at-risk family members.
Genetic Testing is a Matter of Individual Choice
In summary, the choice to undergo genetic testing is a highly personal one. A person's decision depends on various factors, including the perceived preventability and treatability of the disorder and one's ability to make constructive life changes with the information one gains from testing. On one hand, testing can help people make family planning decisions and other life choices, like when to retire or how much money to put away for the future. However, finding out that you are at a higher risk for developing treatable diseases such as cancer or that you carry a gene for an untreatable, fatal disorder such as HD can have negative social consequences. Moreover, it can open the door for potential discrimination. Hopefully, with the recent passage of GINA in the U.S., more Americans will feel comfortable working with physicians and genetic counselors to undergo any genetic tests that contribute to better management of their health care and health choices.
References and Recommended Reading
Allison, M. Industry welcomes Genetic Information Nondiscrimination Act. Nature Biotechnology 26, 596–597 (2008) (link to article)
Creighton, S., et al. Predictive, prenatal, and diagnostic genetic testing for Huntington's disease: The experience in Canada from 1987 to 2000. Clinical Genetics 63, 462–475 (2003)
Forrest, L. E., et al. Communicating genetic information in families: A review of guidelines and position papers. European Journal of Human Genetics 15, 612–618 (2007) doi:10.1038/sj.ejhg.5201822
Gaff, C. L., et al. Process and outcome in communication of genetic information within families: A systematic review. European Journal of Human Genetics 15, 999–1011 (2007) doi:10.1038/sj.ejhg.5201883
Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993)
Kessler, S., et al. Attitudes of persons at risk for Huntington disease toward predictive testing. American Journal of Medical Genetics 26, 259–270 (1987) doi:10.1002/ajmg.1320260204
La Spada, A. R., et al. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nature Genetics 2, 301–304 (1992) (link to article)
Mastromauro, C., et al. Attitudes toward presymptomatic testing in Huntington disease. American Journal of Medical Genetics 26, 271–282 (1987) doi:10.1002/ajmg.1320260205
McConkie-Rosell, A., et al. Living with genetic risk: Effect on adolescent self-concept. American Journal of Medical Genetics 148C, 56–69 (2008)



 Figure 1: Pedigree of an American Huntington's disease family.
Figure 1: Pedigree of an American Huntington's disease family.



























