« Prev Next »
Most books mention that membranes have a typical "lipid bilayer," but why lipids, why should it be a bilayer, and how was this basic structure determined? Although it is now generally taken for granted that membranes are based on the presence of a lipid bilayer, that was not always the case. Early experiments, often by physicists, led to the understanding that the cell membrane was lipid in nature. A key experiment using the Langmuir trough provided the basis for accepting that the membrane is a bilayer and laid the groundwork for the current model of membrane structure.
The Membrane Concept
All cells, prokaryotic or eukaryotic, are surrounded by a plasma membrane. This thin, flexible, and potentially very fragile structure is all that stands between the interior of the cell and the environment. In addition, the insides of eukaryotic cells are subdivided into a series of specialized compartments. Each of these compartments is also surrounded by a membrane (sometimes two or more) that separates the contents from the rest of the cytoplasm. What is it about membranes that make them so critical to the functioning of a cell? The answer comes from the nature of the cell itself and from a couple of basic laws of physics.
Second, we must remember that diffusion is one of the fundamental processes that dictate many of the cell's operations. Molecules will always tend to move from regions of higher concentration to areas of lower concentration. Most cells are surrounded by a dilute aqueous medium, which means that key compounds would, if not prevented, constantly leave the cell. The answer to this problem is to enclose the cytoplasm with a membrane that prevents the free movement of molecules. As described above, a nonpolar material would work well for this membrane because it would not wash away in the surrounding water and would not dissolve water-soluble substances out of the cell.
Discovery of the Lipid Bilayer
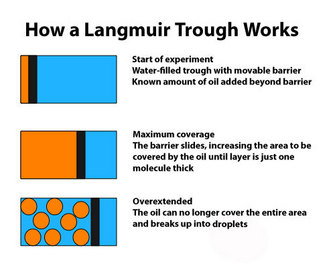
So, if the cell membrane were a lipid, how would it be organized? In 1890, Rayleigh, working on simple oils, showed that they tend to spread over the surface of water. By measuring the original volume of oil and the final area it covered, he was able to calculate the thickness of the film. This initial observation was improved on by the work of Agnes Pockels. Working in her kitchen, and with no formal training, she devised a simple apparatus to quantify the area covered by the oil film. Her apparatus was refined by Langmuir (1917) and is now generally referred to as a Langmuir trough (Figure 2), although it really should be a Pockels trough.
In 1925, Gortner and Grendel performed some key experiments using a Langmuir trough and blood cells (Gortner & Grendel 1925). They were interested in determining the amount of lipid in the membranes of red blood cells. Why use red blood cells? These cells were an excellent choice for this experiment because they have no nucleus or other membrane-bound organelles in the cytoplasm; therefore, any membrane lipids that are found must be those that make up the plasma membrane. First, the scientists extracted the lipids with a variety of solvents, including acetone, from a known number of cells. Then they used the Langmuir trough to determine how large an area the lipids could cover. Because they could measure the actual size (surface area) of a red blood cell and knew approximately how many of those cells they had in their sample, they could calculate the total surface area that would have to be covered by membrane. When the two numbers were compared, it was clear that the amount of lipid they had extracted could cover twice the area needed to enclose all the cells. Why would there be so much? Additional experiments showed that lipids could spontaneously form a bilayer when mixed with water (Figure 1). Together, these observations suggested that there may be a simple explanation for the results with the red blood cells. The plasma membrane of these cells likely consists of a double layer of lipid surrounding each cell.
As it happens, Gortner and Grendel made some errors in their experiment. They failed to completely extract all the lipids from the cells, and they also underestimated the total surface area of the individual red blood cells. However, because these two errors canceled each other out, their final conclusions turned out to be correct, regardless of their miscalculations. Thereafter, the idea of a lipid bilayer became the basis for future models of membrane structure. (Sadava 1993).
Experimental Follow-Up with Microscopy
When the use of electron microscopy started to allow examination of the plasma membrane at high resolution, people noticed that the image clearly showed three layers, not two. In a key paper, Stoeckenius (1962) provided clear pictures of the three-layer structure. He then described in both words and diagrams how the lipid bilayer results in a three-layer image. As it turns out, the inner and outer edges of the bilayer have a different composition than the interior. Under the view of the electron microscope, the outsides of the lipid bilayer show up as two darker layers, whereas the hydrophobic interior stains less densely, thus showing three apparent "layers" (outside layers are represented as blue in Figure 1C).
Summary
The first clues to lipid bilayer structure came from results with red blood cell membranes. The ultimate discovery that the plasma membrane is a lipid bilayer with hydrophobic and hydrophilic properties changed the way this structure was viewed. Its semipermeable and liquid nature provided the groundwork for understanding both its physical and biological properties.
References and Recommended Reading
Edidin, M. Lipids on the frontier: a century of cell-membrane lipids Nature Reviews: Molecular Cell Biology 4: 414–418 (2003).
Gortner, E. & Grendel, F. On bimolecular layers of lipoids on the chromacytes of blood. Journal of Experimental Medicine 41, 439–443 (1925).
Langmuir, I. The constitution and fundamental properties of solids and liquids II: Liquids. Journal of the American Chemical Society 39, 1848–1906 (1917).
Overton, E. The probable origin and physiological significance of cellular osmotic properties. Vierteljahrschrift der Naturforschende gesselschaft 44, 88–135 (1899). In Biological Membrane Structure, trans. Park, R. B. Boston: Little Brown, 1968.
Sadava, D. E. Cell Biology, Organelle Structure and Function. Boston: Jones and Bartlett, 1993.
Stoeckenius, W. Structure of the plasma membrane: An electron-microscope study. Circulation 26, 1066–1069 (1962).
Tanford, C. Ben Franklin Stilled the Waves. Durham, NC: Duke University Press, 1989.



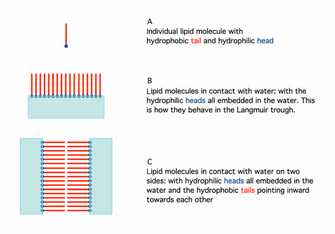
 Figure 1: The orientation of phospholipids in the lipid bilayer.
Figure 1: The orientation of phospholipids in the lipid bilayer.