« Prev Next »

When G. E. Hutchinson argued that evolution is a play taking place on an ecological stage (1965), his point was that ecological interactions — between species and the living (biotic) and non-living (abiotic) aspects of their environment — are the drivers of adaptive evolution. His illustrative examples demonstrate the ubiquity of interspecific interactions that dominate the ecology species: he showed that the foraging niches of organisms as diverse as rhinoceros, birds, cone shells, copepods, and rotifers seem to have diversified in response to competition among similar species within those groups. Beyond competition, most species also interact with other species as a consumer or parasite, a food resource or prey item, or both. Finally, most species are engaged in several kinds of mutualisms with other species, in which both species typically benefit; for example, plants may engage simultaneously in mutually beneficial interactions with fruit-dispersing birds, pollinating insects, nitrogen-fixing bacteria, and mycorrhizal fungi that act like extended root systems in the soil. One potentially dramatic consequence of these interactions can be coevolution, in which the traits of each of two species evolve in response to the traits of the other, resulting in relentlessly reciprocal evolutionary changes in the traits of those interacting species.
Coevolution has been studied for almost as long as the theory of evolution by natural selection has been around. Throughout their writings, Darwin and Wallace both recognized the importance of the coevolutionary process in evolutionary diversification, emphasizing the primacy of species interactions as drivers of adaptive evolutionary change in species. Indeed, it has been argued that Darwin's book on orchids provided the catalyst for all subsequent studies of coevolution and the evolution of extreme specialization (Ghiselin 1984, Thompson 1994). Since Darwin and Wallace, studies on coevolution have shown that species interactions can drive rapid and sustained evolutionary change in species at multiple spatial and temporal scales, generating genetic diversity within populations, leading to adaptive differentiation among populations, and often leading to ecological speciation (Schluter 2009). It has been argued that much of the diversity on earth is a consequence of coevolutionary diversification in species interactions (Thompson 1994, 2005, Ehrlich & Raven 1964). Clearly, studies of coevolution in species interactions can lend insight into the fundamental processes generating and maintaining biodiversity, including genetic and phenotypic diversity within and between species.
What is Coevolution?
Coevolutionary selection between two species within a single local community (i.e., local coevolutionary selection) has the potential to act as a potent evolutionary force on the traits of species by virtue of the feedback driven by its inherent reciprocality. The reciprocality of coevolutionary selection means that the fitnesses of two interacting species depend not only on their own genotypes (and associated traits), but also on each other's genotypes (and traits). This means that there is a genotype by genotype (G x G) interaction on the fitnesses of both species. As a result of this reciprocality, adaptive changes in the traits of one species may trigger subsequent adaptive changes in the traits of the second species, which in turn feed back to cause further adaptive evolution of the first species, and so on. Put another way, the relative fitness of genotypes in one species is context-dependent and the context itself can also evolve (Wade 2003).
For example, the relative fitnesses of different genotypes of a hummingbird and a hummingbird-pollinated plant may depend not only on hummingbird bill shape (which differs among hummingbird genotypes), or on flower morphology (which differs among plant genotypes), but also on the match between the two, generating a G x G interaction for both hummingbird fitness and plant fitness. If hummingbird bill shape adapts in response to selection driven by the average flower morphology in the plant population, the nature of the reciprocal selection on flower morphology (driven by the newly evolved average hummingbird bill shape) changes as a result.
The Crucial Spatial Perspective: Coevolution Occurs in Geographic Mosaics
Although local coevolutionary selection is an important building block of coevolutionary dynamics, studies conducted in the last few decades on coevolution in a wide range of species interactions have demonstrated that coevolution is inherently geographically structured. Specifically, coevolution between two species can progress along very different trajectories in different places, causing trait differences to evolve among different populations of the same species. Because most species are collections of genetically variable populations distributed among environments, and because those environments often differ in abiotic factors and/or community composition, the pattern and strength of natural selection imposed by species on each others' traits are also expected to be highly variable among those environments. This recognition that coevolution is an inherently geographically-structured process has been synthesized by John Thompson as the Geographic Mosaic Theory of Coevolution (GMTC, Thompson 2005).
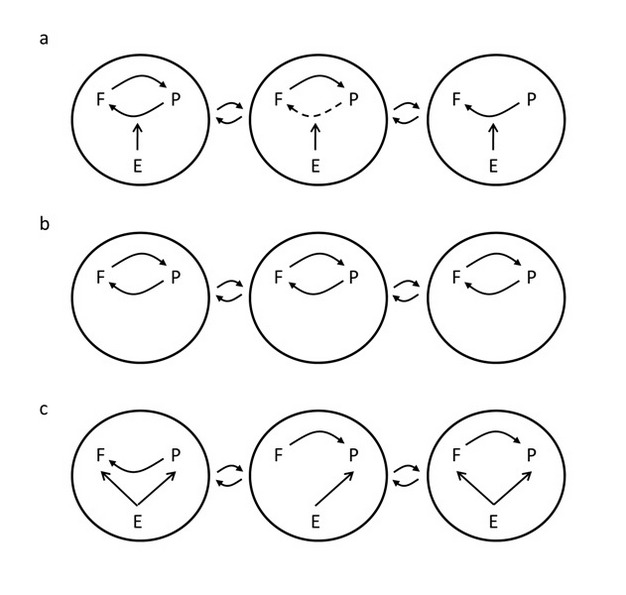
Specifically, the GMTC proposes that coevolution exhibits three inherently geographic characteristics, beyond local coevolutionary selection: coevolutionary hotspots and coldspots, geographic selection mosaics, and trait remixing (Figure 1a). Coevolutionary hotspots are communities in which the pairwise interaction between two species exhibits coevolutionary selection (i.e., there is a G x G interaction on the fitnesses of both species) and these hotspots are expected to be embedded in a geographic matrix of coldspots. In coldspots, selection is not reciprocal, including communities in which only one of the species occurs (i.e., structural coldspots). Geographic selection mosaics occur when a fitness function of coevolutionary selection (dependence of fitness on G x G) differs among environments (E) (i.e., there is a genotype by genotype by environment interaction [G x G x E] for fitness of at least one of the two species). Trait remixing is the suite of processes that potentially influence the geographic distributions of alleles at gene loci underlying coevolving traits (i.e., the geographic structure of G for both species), including mutations, gene flow, genetic drift, and population extinction/recolonization dynamics.
Testing the Importance of Geographic Mosaics of Coevolution
Mathematical modeling studies of geographic mosaics of coevolution have shown how the three key elements of the GMTC can interact to generate a wide variety of outcomes of coevolution, including patterns of specificity, local adaptation/maladaptation, and patterns of genetic variation within and between populations (e.g., Nuismer et al. 2000, Gomulkiewicz et al. 2000, Nuismer 2006). Moreover, empirical studies across a wide range of species interactions — conifers and crossbills (Benkman et al. 2001), snails and trematodes (Lively et al. 2004), camellias and weevils (Toju & Sota 2006), garter snakes and newts (Brodie et al. 2002), and Greya moths that act as both pollinators and seed parasites of Lithophragma plants (Thompson & Cunningham 2002), to name just a few — have provided support for key elements of the theory (reviewed by Thompson 2005 ). For example, Johnson et al. (2010) studied interactions between big bluestem grass (Andropogon gerardii) and its arbuscular mycorrhizal (AM) fungal symbionts from three different populations. They estimated plant and fungal performance in all reciprocal combinations of plant populations (GP), whole AM fungal guilds of species (GF), and sterile soils (E) in a greenhouse experiment. They found a significant GP x GF x E interaction for a key trait of the interaction: the formation of arbuscules, which are the fungal structures in AM plant roots through which nutrients pass. In fact, arbuscule formation was highest in local combinations of plants, fungi, and soils, suggesting that a selection mosaic (different GP x GF interactions for different E) has resulted in local adaptation of plants and fungi to each other in that interaction.
However, it remains challenging to perform such rigorous tests of key phenomena central to the GMTC, especially coevolutionary selection mosaics (Gomulkiewicz et al. 2007), since ideal tests require that all combinations of genotypes of all important interacting species are replicated and studied across multiple field sites or artificial gradients of key environmental variables (Nuismer & Gandon 2008). As a result, many of the necessary experiments have not yet been performed to test the importance of ongoing coevolution and geographic mosaics of coevolution in dominant species interactions, such as those between plants and mycorrhizal fungi (Hoeksema 2010). Furthermore, non-reciprocal adaptation of species to both biotic and abiotic environmental factors has been repeatedly demonstrated to be an important force driving diversification in species (e.g., Bohrer et al. 2003, Saenz-Romero et al. 2006). Thus, a key question that ultimately needs to be addressed in a comprehensive fashion is, what are the relative roles of non-reciprocal selection from biotic and abiotic factors versus the three elements of the GMTC (selection mosaics, hotspots and coldspots, and trait remixing) in driving trait diversification of species?
Broadly speaking, there are at least three hypothetical answers to this question for a particular species interaction (Figure 1, Hoeksema 2010). One hypothesis is that all three of the GMTC elements — hostpots/coldspots, selection mosaics, and trait remixing-are important and interact to play prominent roles in driving diversification within and across communities in which two species occur (Figure 1a). A second hypothesis is that coevolutionary selection is relatively important but is consistent among populations (i.e., true geographic selection mosaics [G x G x E interactions] and hotspots/coldspots are relatively unimportant or uncommon [Figure 1b]). A third hypothesis is that local coevolutionary selection is consistently less important compared to non-reciprocal selection on species by abiotic environmental factors, or by one partner species on the other (Figure 1c). It will be exciting to see whether new individual studies, or perhaps meta-analyses of sets of existing studies, can be designed that distinguish among these possibilities, ultimately helping us to better understand what kinds of drama tend to dominate the evolutionary plays taking place on Hutchinson's ecological stage.
References and Recommended Reading
Benkman, C. W. et al. The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution 55, 282–294 (2001).
Bohrer, G. et al. Effects of different Kalahari-desert VA mycorrhizal communities on mineral acquisition and depletion from the soil by host plants. Journal of Arid Environments 55, 193–208 (2003).
Brodie, E. D. Jr. et al. The evolutionary response of predators to dangerous prey: Hotspots and coldspots in the geographic mosaic of coevolution between newts and snakes. Evolution 56, 2067–2082 (2002).
Ehrlich, P. R. & Raven, P. H. Butterflies and plants: A study in coevolution. Evolution 18, 586–608 (1964).
Ghiselin, M. T. " Pages xi–xix" in The Various Contrivances by Which Orchids are Fertilized by Insects, by C. Darwin, Reprint of 2nd Edition, 1877. Chicago, IL: University of Chicago Press, 1984.
Gomulkiewicz, R. et al. Hot spots, cold spots, and the geographic mosaic theory of coevolution. The American Naturalist 156, 156–174 (2000).
————— Dos and don'ts of testing the geographic mosaic theory of coevolution. Heredity 98, 249–258 (2007).
Hoeksema, J. D. Tansley Review. Ongoing coevolution in mycorrhizal interactions. New Phytologist 187, 286–300 (2010).
Johnson, N. C. et al. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proceedings of the National Academy of Sciences of the United States of America 107, 2093–2098 (2010).
Lively, C. M. et al. Host sex and local adaptation by parasites in a snail-trematode interaction. The American Naturalist 164, S6–S18 (2004).
Nuismer, S. L. Parasite local adaptation in a geographic mosaic. Evolution 60, 24–30 (2006).
Nuismer, S. L. & Gandon, S. Moving beyond common-garden and transplant designs: Insight into the causes of local adaptation in species interactions. The American Naturalist 171, 658–668 (2008).
Nuismer, S. L. et al. Coevolutionary clines across selection mosaics. Evolution 54, 1102–1115 (2000).
Saenz-Romero, C. et al. Altitudinal genetic variation among Pinus oocarpa populations in Michoacan, Mexico: Implications for seed zoning, conservation, tree breeding and global warming. Forest Ecology and Management 229, 340–350 (2006).
Schluter, D. Evidence for ecological speciation and its alternative. Science 323, 737–741 (2009).
Thompson, J. N. The Coevolutionary Process. Chicago, IL: University of Chicago Press, 1994.
Thompson, J. N. The Geographic Mosaic of Coevolution. Chicago, IL: University of Chicago Press, 2005.
Thompson, J. N. & Cunningham, B. M. Geographic structure and dynamics of coevolutionary selection. Nature 417, 735–738 (2002).
Toju, H. & Sota, T. Imbalance of predator and prey armament: Geographic clines in phenotypic interface and natural selection. The American Naturalist 167, 105–117 (2006).
Wade, M. J. Community genetics and species interactions. Ecology 84, 583–585 (2003).






























