« Prev Next »

Corals reefs are communities of organisms in which corals (members of the phylum Cnidaria) provide the dominant structural elements. They are known to need a range of specific conditions, such as clear agitated waters of normal salinity, free from sediment, and a temperature between about 18 and 30oC. Within the structure of the coral reefs, members of almost every phyla of plants and animals live and die, adding particles and organic material to the sediment, and contributing to the efficient functioning of the coral reef ecosystem. It is estimated that about 25% of all marine species are found in coral reefs. The paradox of coral reefs is that, although they are highly diverse ecosystems, contain an abundance of organic material, and are highly productive, they exist in essentially oligotrophic environments. The analogy is often drawn to tropical rainforests, which are also extremely diverse and exist in nutrient poor conditions. The key to the existence of coral reefs rests in the rapid recycling of nutrients between all the components of the community. If this balance is disturbed — for example by the addition of nutrients in excess from an outside source — the ecosystem frequently declines.
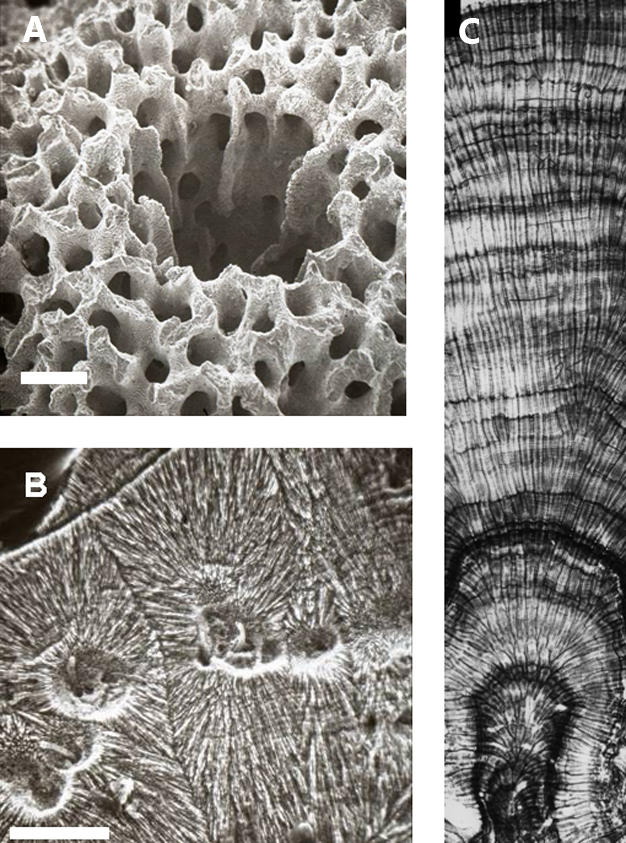
The coral organism itself is relatively primitive, possessing only two basic cellular layers, separated by a non-cellular layer known as the mesoglea, and possessing a rudimentary nervous system (Figure 1). The basic unit of a coral is known as a polyp, essentially an opening surrounded by tentacles into which food, captured by tentacles possessing stinging cells (Cnidoblasts), is placed and digested. Undigested waste is released through the same opening. Collections of interconnected polyps make up the coral colony, although there are some corals that consist of only one polyp. The polyps rest on a skeleton made calcium carbonate secreted by one of the cellular layers known as the calicoblastic epithelium. The coral organism is thought to manipulate the carbonate chemistry within a small extracellular space between the epithelium and the skeleton by extracting hydrogen ions, thus raising pH and excreting calcium ions. This process raises the carbonate saturation and a form of calcium carbonate — normally aragonite — is precipitated. The delicate coral skeleton is shaped by the coral altering the dimensions and the direction of the extracellular space. The coral organism inhabits only the uppermost floor of the skeleton, continually building new layers, and moving upwards. The process is akin to the construction of an apartment building, but with the coral tissue only occupying the top floor. As successive floors and walls are constructed, the coral moves upwards leaving the lower portion of the skeleton unoccupied. This vacancy is, however, short-lived, as a variety of organisms rapidly bore into the skeleton, including algae, fungi, sponges, and mollusks. Such organisms weaken the structure of the skeleton, eventually causing the corals to collapse under the weight of the new growth. The boring process essentially limits the life span of the coral. Corals that form low-density skeletons are more easily bioeroded, and hence have a shorter life span compared to corals that form denser structures. There are many types of corals: massive boulder corals, platy corals, branching corals, and solitary corals. Branching corals are generally fast growing, showing extension rates of 2–3 cm per year, and frequently forming massive thickets. In contrast, massive corals form larger boulder type structures, often with annual density bands (Figure 2). These types of corals can be cored and dated by counting the annual growth bands. When the extension rate and density are combined, the calcification rate can be determined. Hence extension and calcification rates of certain types of corals can be measured over hundreds of years, providing a picture of the health of the coral and the coral reef community (Dodge et al. 1977, Dodge et al. 1984, Helmle et al. 2011). The chemical composition of the coral skeleton can also be analyzed within the age structure provided by the density bands. For example, the ratio of the stable isotopes of oxygen (18O and 16O) varies as a function of temperature and salinity (Swart et al. 1980, Weber et al. 1972). Other elements, such as strontium, vary only with respect to temperature (Weber 1973). By combining these so-called ‘geochemical proxies,' scientists can unravel a long term history of salinity and water temperature. In addition, numerous other elements can be analyzed within the chronological framework providing long term histories of climate and anthropogenic influence (Nozaki et al. 1978, Fairbanks et al. 1979, McCulloch et al. 1994, Swart et al. 1996a, 1996c, 2010, Hendy et al. 2002, Marion et al. 2006).

Modern shallow water coral reefs are dominated by corals that show a symbiotic association with a unicellular dinoflagellate algae (photosymbionts), which lives within the endodermis of the coral. These are often referred to as zooxanthellae. These algae provide photosynthate to the coral, hence promoting growth (Pearse et al. 1971). In fact, many corals are autotrophic. In return, it is believed that the corals provide inorganic nitrogen to the algae, enabling them to survive in essentially oligotrophic conditions. These processes can be tracked using the stable isotope of carbon (13C/12C) (Swart 1983, Swart et al. 1996b, Grottoli et al. 1999). The photosymbionts need light, limiting reefs with such corals to the portion of the ocean receiving sunlight (the photic zone). In addition to corals containing photosymbionts, there are also corals that normally exist without symbionts (non-symbiotic). These can occur in shallow water, but are abundant in deeper waters below the photic zone, where they are the main structural elements of deep-water reefs. Deep-water reefs are largely unknown and unseen, but are estimated to be volumetrically more important than the shallow-water structures most people are familiar with. A third category or corals can exist normally in either state. Corals that form reefs — whether they contain photosymbionts or not — are called hermatypic corals. Corals that exist as solitary individuals are ahermatypic corals. The presence of the photosymbionts in the coral tissue positively affects the growth rate of the coral (Goreau 1959). It has been postulated that the emergence of corals as the main structural element in reefs, during the Triassic, is a direct result of the development of this symbiosis (Stanley 1981, Stanley et al. 1995). Under adverse conditions, induced by high temperatures or high insolation, corals expel their zooxanthellae. The corals loose their colour causing a condition known as bleaching. The loss dramatically reduces growth rates of the affected corals, and death often follows. Although the history of bleaching in corals is uncertain, there is certainly a perception that the instances of bleaching have increased over the past several decades, an increase believed to result from the steady rise in sea surface temperatures and/or the increased instances of climatic phenomena such as El Niňo (Glynn 1989). The phenomenon of bleaching is not always uniform in all corals in the affected reef, a feature believed to be related to different types or lineages of zooxanthellae. For example, in a bleaching episode that affected the Eastern Pacific, it was found that a large portion of a certain species bleached during an El Niňo event. Those specimens that did not bleach were found to contain a different lineage of zooxanthellae (Baker et al. 2004). The trade off is that the more resistant clade is present in corals with lower growth rates, and thus, under normal conditions, these corals would be at a disadvantage compared to the corals with the lineage more susceptible to bleaching. Because bleaching alters the flow of carbon between the coral and the zooxanthellae, there was hope that past records of bleaching episodes might be recorded in the skeleton by the stable carbon isotope ratio. However, because the main affect of bleaching is to cause a reduction in skeletal accretion, and the skeleton is the vehicle for recording the change in the carbon isotope value, it has proved difficult to record geochemical evidence of bleaching (Leder et al. 1991). Frequently the most noticeable signal of bleaching is a wider than normal dense band in the skeleton. In some instances this may result in the coral extension being so reduced that it effectively misses a year of normal growth (Figure 2c).
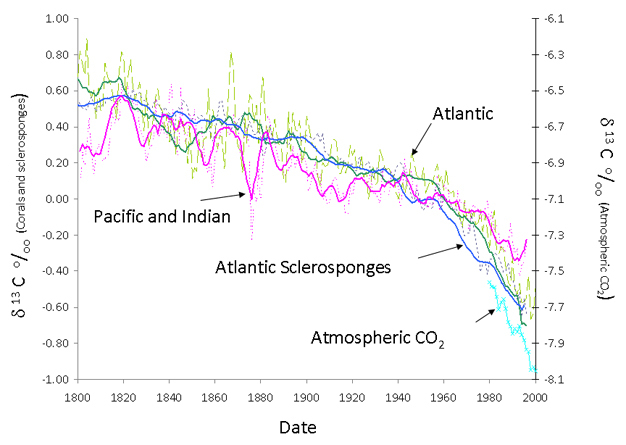
While bleaching of corals may be of growing concern as a result of the global warming of the oceans, both shallow and deep coral reefs are under growing threats from a variety of anthropogenic factors. These threats are both locally specific and global in nature. Local effects on reefs usually result from overexploitation, resulting from overfishing, pollution, or exploitation of the reefs for natural resources. Overfishing is particularly pernicious in developing countries where literally all fish are cleared from reefs, usually to sustain the diet of poor local inhabitants. Removal of fish disrupts the delicate balance within the reef. Herbivorous fish play a crucial role in conjunction with invertebrates in keeping the reefs clear of algae. In the absence of herbivory, surfaces on corals reefs can become inundated with fleshy algae competing with juvenile corals for attachment space and interacting with and overgrowing corals. Reduced reef fish are a greater threat in the face of changes in the population of other reef organisms such as sea urchins. In the Caribbean, for example, large decreases in the number of grazing sea urchins have been implicated in the phase shift from coral to algae (Porter 1992). Land-based sources of pollution in many forms are also of concern. Coral reefs generally grow in waters with low sediment input. Dirty water blocks light from reaching the photosymbionts, and particulates settle on the coral surfaces which they have trouble removing. For these reasons coral reefs are generally not found close to the mouths of major rivers, which deliver large amounts of sediments and are adversely affected by changes in local land use. Inputs of external nutrients in the form of dissolved inorganic nitrogen disrupts the delicate nutrient balance in the coral reef, promoting the growth of fleshy algae, and disrupting the flow of photosynthate from photosymbionts to the corals. If corals reefs were not under enough threats from local use, CO2 from anthropogenic sources is causing an increase in atmospheric concentration. This in turn enters into the oceans causing an increase in acidity (Langdon et al. 2000) and lowers the saturation of the mineral aragonite, which forms the skeletons of the corals. Although the level of saturation in the surface oceans is still significantly higher than the level at which dissolution would take place, and there is no evidence from cores taken from coral skeletons that decreases in calcification have taken place (Helmle et al. 2011), it is thought that even a small decrease in saturation causes the corals to work harder to form their skeletons. Deeper coral reefs may be under greater threat as the waters in these areas are already at reduced levels of saturation. The history of the addition of fossil fuel CO2 to the oceans is readily visible as a decrease in the 13C/12C ratio of coral skeletons (Swart et al. 2010) (Figure 3).
Over the long term, as coral reefs continue to build themselves up, the framework of the coral reef is filled with sediment and cemented with calcite and aragonite. If sea level changes, the reefs become exposed to corrosive rainwater that dissolves the unstable aragonite and precipitates calcite. This process converts the reef into hard, dense limestone, a material frequently used as building material. Corals which only formed in the past 100,000 years are quickly altered. Although the skeletons can no longer be used for interpreting paleoenvironments, most of the organisms that formed the reef can still be identified, and ecological studies can be carried out (Klaus et al. 2011) (Figure 4). In certain circumstances, the process of converting aragonite to calcite can be retarded, and the coral skeletons preserved. For example if the corals are exposed to a dry environment, their alteration to calcite will be reduced (Figure 5). Under these circumstances, and ones in which corals are preserved in marine dominated fluids, the geochemical record can be successively measured and interpreted in terms of changes in global climate (Greer et al. 2006, 2009).


Coral reefs are very much the rainforests of the oceans. Not only are they diverse communities, but the coral skeletons — like trees — potentially contain long term records of climate which can be interpreted in terms of temperature, salinity, and anthropogenic impact. However, while coral reefs can be compared to rainforests, they are also the canaries of the sea, being very sensitive to small changes in the chemical and physical condition of their environment. Modern coral reefs — whether deep or shallow — are under attack from a wide range of local and globally distributed anthropogenic factors.
References and Recommended Reading
Baker, A. C. et al. Corals' adaptive response to climate change. Nature 430, 741 (2004).
Dodge, R. E. & Vaisrys, J. R. Coral populations and growth patterns: Responses to sedimentation and turbidity associated with dredging. Journal of Marine Research 35, 715-730 (1977).
Dodge, R. E. & Brass, G. W. Skeletal extension, density and calcification of the coral, Montastrea annularis: St. Croix, U.S. Virgin Islands. Bulletin of Marine Science 34, 288-307 (1984).
Fairbanks, R. G. & Dodge, R. E. Annual periodicity of 18O/16O and 13C/12C ratios in the coral Montastraea annularis. Geochimica et Cosmochimica Acta 43, 1009-1020 (1979).Glynn, P. W. El Niño-Southern Oscillation 1982-83: Nearshore population, community, and ecosystem responses. Annual Review of Ecology and Systematics 19, 309-345 (1988).
Goreau, T. F. The physiology of skeleton formation in corals. I. A method for measuring the rate of calcium deposition by corals under different conditions. The Biological Bulletin 116, 59-75 (1959).
Greer, L. & Swart, P. K. Decadal cyclicity of regional mid-Holocene precipitation: Evidence from Dominican coral proxies. Paleoceanography 21, PA2020 (2006).
Greer, L. et al. How vulnerable is Acropora cervicornis to environmental change? Lessons from the early to middle Holocene. Geology 37, 263-266 (2009).
Grottoli, A. G. & Wellington, G. M. Effect of light and zooplankton on skeletal d13C values in the eastern Pacific corals Pavona clavus and Pavona gigantea. Coral Reefs 18, 29-41 (1999).
Helmle, K. P. et al. Growth rates of Florida corals from 1937 to 1996 and their response to climate change. Nature Communications 2, 215 (2011).
Hendy, E. J. et al. Abrupt decrease in tropical Pacific sea surface salinity at end of Little Ice Age. Science 295, 1511-1514 (2002).
Klaus, J. S. et al. Rise and fall of Pliocene free-living corals in the Caribbean. Geology 39, 375-378 (2011).
Langdon, C. et al. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochemical Cycles 14, 639-654 (2000).
Leder, J. J. et al. The effect of prolonged "bleaching" on skeletal banding and stable isotopic composition in Montastrea annularis. Coral Reefs 10, 19-27 (1991).
Marion, G. S. et al. Nitrogen isotopes (d15N) in coral skeleton: Assessing provenance in the Great Barrier Reef lagoon. Geochimica et Cosmochimica Acta 70, A392-A392 (2006).
McCulloch, M. et al. A high resolution Sr/Ca and 18 O coral record from the Great Barrier Reef, Australia, and the 1982-1983 El Niño. Geochimica et Cosmochimica Acta 58, 2747-2754 (1994).
Nozaki, Y. et al. A 200 year record of carbon-13 and carbon-14 variation in a Bermuda coral. Geophysical Research Letters 5, 825-828 (1978).
Pearse, V. B. & Muscatine, L. Role of symbiotic algae (zooxanthellae) in coral calcification. Biological Bulletin 141, 350-363 (1971).
Porter, J. Quantification of loss and change in the Floridian reef coral populations. American Zoologist 32, 625-640 (1992).
Stanley, G. D. Jr. Early history of scleractinian corals and its geological consequences. Geology 9, 507-511 (1981).
Stanley, G. D. Jr. & Swart, P. K. Evolution of the coral-zooxanthellae symbiosis during the Triassic: A geochemical approach. Paleobiology 21, 179-199 (1995).
Swart, P. K. Carbon and oxygen isotope fractionation in scleractinian corals: A review. Earth-Science Reviews 19, 51-80 (1983).
Swart, P. K. & Coleman, M. L. Isotopic data for scleractinian corals explain their palaeotemperature uncertainties. Nature 283, 557-559 (1980).
Swart, P. K. et al. A 240-year stable oxygen and carbon isotopic record in a coral from South Florida: Implications for the prediction of precipitation in southern Florida. Palaios 11, 362-375 (1996a).
Swart, P. K. et al. The origin of variations in the isotopic record of scleractinian corals: II Carbon. Geochimica et Cosmochimica Acta 60, 2871-2886 (1996b).
Swart, P. K. et al. The stable oxygen and carbon isotopic record from a coral growing in Florida Bay: A 160 year record of climatic and anthropogenic influence. Palaeogeography, Palaeoclimatology, Palaeoecology 123, 219-237 (1996c).






























