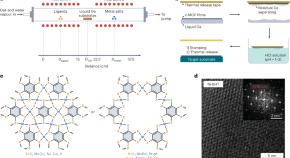
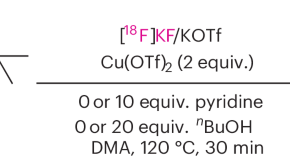
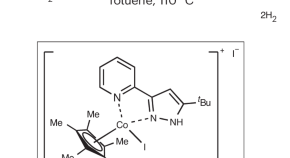
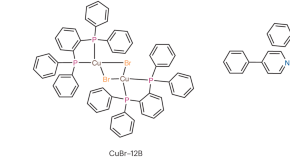
Featured

Advertisement
-
-

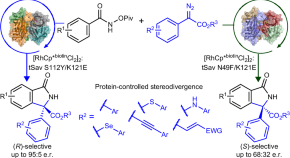
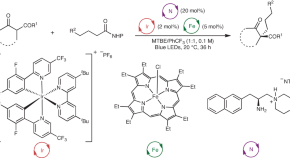
Artificial metalloenzyme for the enantiodivergent synthesis of isoindolones
An artificial metalloenzyme based on streptavidin with a biotinylated Rh(III) cofactor provides enantioselective access to various isoindolones with different functional groups. Rational engineering of the streptavidin scaffold reverses the stereoselectivity, offering an enantiodivergent method for the synthesis of isoindolones.
-
-