Abstract
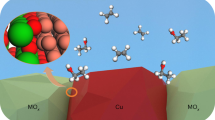
Alloying of metals can be used to optimize intermediate binding during electrocatalysis but challenges remain in overcoming thermodynamic atomic miscibility in alloys. Here we report a coordination-controlled metal alloy in which copper clusters are spatially dispersed in a crystalline silver lattice to promote the electrochemical reduction of CO2 to ethanol. The synergistic interactions between Cu–Cu sites and Cu–Ag interfaces achieve highly selective hydrocarbon and oxygenate production by strengthening and diversifying the binding of *CO intermediates on terrace and defect sites. To control atomic coordinates beyond the miscibility limit and optimize the catalyst microstructure, sacrificial elements are incorporated with thermodynamically guided compositions to form intermetallic compounds. The sacrificial elements are then selectively dealloyed. Using a membrane electrode assembly, ethylene-selective production on copper catalysts (Faradaic efficiency, 69.6 ± 1.3%; full cell efficiency, 23.5%) is steered to ethanol-selective production on the supersaturated Ag–Cu solid-solution catalyst (Faradaic efficiency, 40.4 ± 2.4%; full cell efficiency, 14.4%). Metallurgy-designed catalyst fabrication enables the efficient chemical manufacturing of either hydrocarbons or oxygenates and offers guidelines for catalyst design principles.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the articles and its Supplementary Information.
References
Meys, R. et al. Achieving net-zero greenhouse gas emission plastics by a circular carbon economy. Science 374, 71–76 (2021).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Razmjoo, A. et al. A technical analysis investigating energy sustainability utilizing reliable renewable energy sources to reduce CO2 emissions in a high potential area. Renewable Energy 164, 46–57 (2021).
Sa, Y. J. et al. Catalyst–electrolyte interface chemistry for electrochemical CO2 reduction. Chem. Soc. Rev. 49, 6632–6665 (2020).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Bushuyev, O. S. et al. What should we make with CO2 and how can we make it? Joule 2, 825–832 (2018).
Kibria, M. G. et al. Electrochemical CO2 reduction into chemical feedstocks: from mechanistic electrocatalysis models to system design. Adv. Mater. 31, 1807166 (2019).
Li, L., Li, X., Sun, Y. & Xie, Y. Rational design of electrocatalytic carbon dioxide reduction for a zero-carbon network. Chem. Soc. Rev. 51, 1234–1252 (2022).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2020).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Pan, J. et al. Hierarchical and ultrathin copper nanosheets synthesized via galvanic replacement for selective electrocatalytic carbon dioxide conversion to carbon monoxide. Appl. Catal. B. 255, 117736 (2019).
Hao, Q. et al. Nickel dual-atom sites for electrochemical carbon dioxide reduction. Nat. Synth. 1, 719–728 (2022).
Xia, C. et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4, 776–785 (2019).
Fan, L., Xia, C., Zhu, P., Lu, Y. & Wang, H. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 11, 3633 (2020).
Chen, S. et al. Engineering water molecules activation center on multisite electrocatalysts for enhanced CO2 methanation. J. Am. Chem. Soc. 144, 12807–12815 (2022).
Li, Y. et al. Promoting CO2 methanation via ligand-stabilized metal oxide clusters as hydrogen-donating motifs. Nat. Commun. 11, 6190 (2020).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
García de Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−1. Science 367, 661–666 (2020).
Ozden, A. et al. Cascade CO2 electroreduction enables efficient carbonate-free production of ethylene. Joule 5, 706–719 (2021).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Zhu, P. & Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 4, 943–951 (2021).
Karapinar, D., Creissen, C. E., Rivera de la Cruz, J. G., Schreiber, M. W. & Fontecave, M. Electrochemical CO2 reduction to ethanol with copper-based catalysts. ACS Energy Lett. 6, 694–706 (2021).
Todorova, T. K., Schreiber, M. W. & Fontecave, M. Mechanistic understanding of CO2 reduction reaction (CO2RR) toward multicarbon products by heterogeneous copper-based catalysts. ACS Catal. 10, 1754–1768 (2020).
Li, F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 3, 75–82 (2020).
Schneider, C. R., Manesis, A. C., Stevenson, M. J. & Shafaat, H. S. A photoactive semisynthetic metalloenzyme exhibits complete selectivity for CO2 reduction in water. Chem. Commun. 54, 4681–4684 (2018).
Can, M., Armstrong, F. A. & Ragsdale, S. W. Structure, function, and mechanism of the nickel metalloenzymes, CO dehydrogenase, and acetyl-CoA synthase. Chem. Rev. 114, 4149–4174 (2014).
Zhi, X., Vasileff, A., Zheng, Y., Jiao, Y. & Qiao, S.-Z. Role of oxygen-bound reaction intermediates in selective electrochemical CO2 reduction. Energy & Environ. Sci. 14, 3912–3930 (2021).
Zhou, Y. et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 10, 974–980 (2018).
Ting, L. R. L. et al. Enhancing CO2 electroreduction to ethanol on copper–silver composites by opening an alternative catalytic pathway. ACS Catal. 10, 4059–4069 (2020).
Li, Y. C. et al. Binding site diversity promotes CO2 electroreduction to ethanol. J. Am. Chem. Soc. 141, 8584–8591 (2019).
Morales-Guio, C. G. et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 1, 764–771 (2018).
Baek, Y. et al. Electrochemical carbon dioxide reduction on copper–zinc alloys: ethanol and ethylene selectivity analysis. J. Mater. Chem. A 10, 9393–9401 (2022).
Ren, D. et al. Atomic layer deposition of ZnO on CuO enables selective and efficient electroreduction of carbon dioxide to liquid fuels. Angew. Chem. Int. Ed. 58, 15036–15040 (2019).
Zhang, T. et al. Highly selective and productive reduction of carbon dioxide to multicarbon products via in situ CO management using segmented tandem electrodes. Nat. Catal. 5, 202–211 (2022).
Gu, Z. et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 5, 429–440 (2021).
Arafa, M. K. I. A calculation of the solubility limits of the copper–silver system. Proc. Phys. Soc. B 62, 238 (1949).
El Shayeb, H., El Wahab, F. & El Abedin, S. Z. Electrochemical behaviour of Al, Cu, and Al–Cu alloys in NaOH and HCl solutions. Br. Corros. J. 34, 37–43 (1999).
Cantor, B., Kim, W., Bewlay, B. & Gillen, A. Microstructure—cooling rate correlations in melt-spun alloys. J. Mater. Sci. 26, 1266–1276 (1991).
Li, Y. Bulk metallic glasses: eutectic coupled zone and amorphous formation. JOM 57, 60–63 (2005).
Gill, S. & Kurz, W. Rapidly solidified Al–Cu alloys—I. experimental determination of the microstructure selection map. Acta Metallur. Mater. 41, 3563–3573 (1993).
Parida, S. et al. Volume change during the formation of nanoporous gold by dealloying. Phys. Rev. Lett. 97, 035504 (2006).
Gao, P. et al. Defects evolution in nanoporous Au (Pt) during dealloying. Scr. Mater. 113, 68–70 (2016).
Kim, C. et al. Cu/Cu2O interconnected porous aerogel catalyst for highly productive electrosynthesis of ethanol from CO2. Adv. Funct. Mater. 31, 2102142 (2021).
Nguyen, T. N. et al. Electrochemical CO2 reduction to ethanol:from mechanistic understanding to catalyst design. J. Mater. Chem. A 9, 12474–12494 (2021).
Chang, C.-J. et al. Dynamic reoxidation/reduction-driven atomic interdiffusion for highly selective CO2 reduction toward methane. J. Am. Chem. Soc. 142, 12119–12132 (2020).
Chang, C.-J. et al. Quantitatively unraveling the redox shuttle of spontaneous oxidation/electroreduction of CuOx on silver nanowires using in situ X-ray absorption spectroscopy. ACS Cent. Sci. 5, 1998–2009 (2019).
Deng, Y. & Yeo, B. S. Characterization of electrocatalytic water splitting and CO2 reduction reactions using in situ/operando Raman spectroscopy. ACS Catal. 7, 7873–7889 (2017).
Zhan, C. et al. Revealing the CO coverage-driven C–C coupling mechanism for electrochemical CO2 reduction on Cu2O nanocubes via operando Raman spectroscopy. ACS Catal. 11, 7694–7701 (2021).
An, H. et al. Sub‐second time‐resolved surface‐enhanced Raman spectroscopy reveals dynamic CO intermediates during electrochemical CO2 reduction on copper. Angew. Chem. Int. Ed. 60, 16576–16584 (2021).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Grimme, S. Semiempirical GGA‐type density functional constructed with a long‐range dispersion correction. J. Comput. Chem. 27, 1787–1799 (2006).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Acknowledgements
This research was supported by the Creative Materials Discovery Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT (MSIT) and Future Planning (2017M3D1A1040689 and 2019M3D1A1079215); the program of Carbon to X technology development for the production of useful substances (NRF-2020M3H7A1098376) through the National Research Foundation of Korea (NRF), funded by the Korean government (Ministry of Science and ICT (MSIT)); and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2018M3A7B8060601 and NRF-2021R1C1C1013784). Material characterization, including X-ray diffraction, SEM and TEM analysis, was supported by the Research Institute of Advanced Materials, Institute of Engineering Research and the National Center for Interuniversity Research Facilities in Seoul National University. ICP-AES and NMR analysis were supported by the National Instrumentation Center for Environmental Management. XPS analysis was supported by the Korea Institute of Ceramic Engineering and Technology. Y.-C.J. acknowledges support from the Supercomputing Center/Korea Institute of Science and Technology Information with supercomputing resources (KSC-2021-RND-0010). Experiments at PLS-II were supported in part by MSIT and POSTECH.
Author information
Authors and Affiliations
Contributions
Y.-C.J., E.S.P. and D.-H.N. designed and supervised the overall project. J.-Y.K., H.S.A. and I.K. conceived the idea and carried out the experiments. J.-Y.K. and I.K. conducted the dealloying, characterization and electrochemical experiments. H.S.A. designed and fabricated the alloy. D.H. conducted the DFT calculations. J.J. and H.G.K. carried out the TEM analysis. H.K. and G.K. contributed to the CO2RR performance evaluation and thermodynamic calculation, respectively. M.K.K. and W.H.R. contributed to the alloy fabrication and CCT diagram construction. T.L. and S.G. performed XAS and Raman analysis. G.-D.L. and M.K. supervised the DFT calculations and TEM analysis, respectively. All authors discussed the results and contributed to the paper writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Zhiliang Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–74 and Tables 1–3.
Supplementary Data 1
Electrochemical CO2 reduction performance and DFT calculation
Source data
Source Data Fig. 1
Source data for DFT and thermochemical calculations.
Source Data Fig. 2
Source data for thermochemical calculation, CCT diagram and electrochemical CO2 reduction performance.
Source Data Fig. 2
Source data for ternary phase diagrams.
Source Data Fig. 3
Source data for phase fraction and atomic composition.
Source Data Fig. 4
Source data for phase and state analysis.
Source Data Fig. 5
Source data for electrochemical CO2 reduction performance.
Source Data Fig. 6
Source data for Raman analysis and DFT calculation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, JY., Ahn, H.S., Kim, I. et al. Selective hydrocarbon or oxygenate production in CO2 electroreduction over metallurgical alloy catalysts. Nat. Synth 3, 452–465 (2024). https://doi.org/10.1038/s44160-023-00449-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00449-6