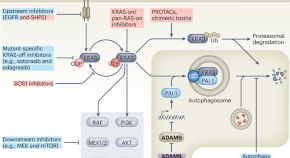
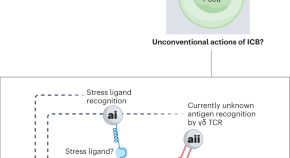
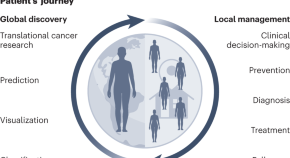
Featured
Advertisement
-
-

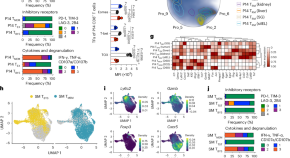
Circadian regulation of cancer stem cells and the tumor microenvironment during metastasis
Barker and colleagues discuss the interplay between circadian rhythm, the tumor microenvironment and stem cells and how these are linked to metastasis as well as how these interactions could be clinically relevant.
-
-
Trending - Altmetric
-
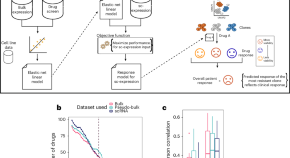
PERCEPTION predicts patient response and resistance to treatment using single-cell transcriptomics of their tumors
-
The selective prolyl hydroxylase inhibitor IOX5 stabilizes HIF-1α and compromises development and progression of acute myeloid leukemia
-
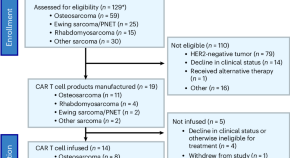
Autologous HER2-specific CAR T cells after lymphodepletion for advanced sarcoma: a phase 1 trial
-
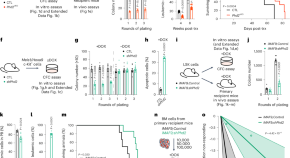
Single-cell multiomic dissection of response and resistance to chimeric antigen receptor T cells against BCMA in relapsed multiple myeloma