Abstract
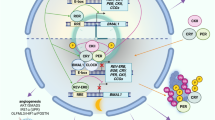
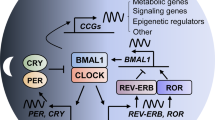
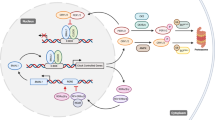
The circadian clock regulates daily rhythms of numerous physiological activities through tightly coordinated modulation of gene expression and biochemical functions. Circadian disruption is associated with enhanced tumor formation and metastasis via dysregulation of key biological processes and modulation of cancer stem cells (CSCs) and their specialized microenvironment. Here, we review how the circadian clock influences CSCs and their local tumor niches in the context of different stages of tumor metastasis. Identifying circadian therapeutic targets could facilitate the development of new treatments that leverage circadian modulation to ablate tumor-resident CSCs, inhibit tumor metastasis and enhance response to current therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Roenneberg, T. & Merrow, M. The circadian clock and human health. Curr. Biol. 26, R432–R443 (2016).
Hastings, M. H., Maywood, E. S. & Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 19, 453–469 (2018).
Koronowski, K. B. & Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 371, eabd0951 (2021).
Laothamatas, I., Rasmussen, E. S., Green, C. B. & Takahashi, J. S. Metabolic and chemical architecture of the mammalian circadian clock. Cell Chem. Biol. 30, 1033–1052 (2023).
Straif, K. et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 8, 1065–1066 (2007).
Oshima, T. et al. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol. Rep. 25, 1439–1446 (2011).
Huisman, S. A. et al. Disruption of clock gene expression in human colorectal liver metastases. Tumour Biol. 37, 13973–13981 (2016).
Liu, K. et al. Reprogramming the tumor microenvironment by genome editing for precision cancer therapy. Mol. Cancer 21, 98 (2022).
Oskarsson, T., Batlle, E. & Massague, J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 14, 306–321 (2014).
Prasetyanti, P. R. & Medema, J. P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 16, 41 (2017).
Korkaya, H. et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol. Cell 47, 570–584 (2012).
Sharma, V. P., Anderson, N. T. & Geusz, M. E. Circadian properties of cancer stem cells in glioma cell cultures and tumorspheres. Cancer Lett. 345, 65–74 (2014).
Schmitt, K. et al. Circadian control of DRP1 activity regulates mitochondrial dynamics and bioenergetics. Cell Metab. 27, 657–666 (2018).
Patke, A., Young, M. W. & Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 21, 67–84 (2020).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Narasimamurthy, R. & Virshup, D. M. The phosphorylation switch that regulates ticking of the circadian clock. Mol. Cell 81, 1133–1146 (2021).
Wang, Y., Guo, H. & He, F. Circadian disruption: from mouse models to molecular mechanisms and cancer therapeutic targets. Cancer Metastasis Rev. 42, 297–322 (2023).
Dibner, C., Schibler, U. & Albrecht, U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549 (2010).
Ray, S. et al. Circadian rhythms in the absence of the clock gene Bmal1. Science 367, 800–806 (2020).
Ness-Cohn, E., Allada, R. & Braun, R. Comment on ‘Circadian rhythms in the absence of the clock gene Bmal1’. Science 372, eabe9230 (2021).
Ray, S. et al. Response to comment on ‘Circadian rhythms in the absence of the clock gene Bmal1’. Science 372, eabf1930 (2021).
Kondratov, R. V., Kondratova, A. A., Gorbacheva, V. Y., Vykhovanets, O. V. & Antoch, M. P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 20, 1868–1873 (2006).
Kettner, N. M. et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell 30, 909–924 (2016).
Puram, R. V. et al. Core circadian clock genes regulate leukemia stem cells in AML. Cell 165, 303–316 (2016).
Wu, J. et al. Disruption of the clock component Bmal1 in mice promotes cancer metastasis through the PAI-1–TGF-β–myoCAF-dependent mechanism. Adv. Sci. 10, e2301505 (2023).
Hadadi, E. et al. Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat. Commun. 11, 3193 (2020).
Papagiannakopoulos, T. et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 24, 324–331 (2016).
Jiang, W. et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 371, 314–325 (2016).
Miki, T., Matsumoto, T., Zhao, Z. & Lee, C. C. p53 regulates Period2 expression and the circadian clock. Nat. Commun. 4, 2444 (2013).
El-Athman, R. et al. The Ink4a/Arf locus operates as a regulator of the circadian clock modulating RAS activity. PLoS Biol. 15, e2002940 (2017).
Filipski, E. et al. Effects of light and food schedules on liver and tumor molecular clocks in mice. J. Natl Cancer Inst. 97, 507–517 (2005).
Wu, M. et al. Experimental chronic jet lag promotes growth and lung metastasis of Lewis lung carcinoma in C57BL/6 mice. Oncol. Rep. 27, 1417–1428 (2012).
Chen, J. et al. Downregulation of the circadian rhythm regulator HLF promotes multiple-organ distant metastases in non-small cell lung cancer through PPAR/NF-κb signaling. Cancer Lett. 482, 56–71 (2020).
Yang, X. et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 126, 801–810 (2006).
Tognini, P. et al. Reshaping circadian metabolism in the suprachiasmatic nucleus and prefrontal cortex by nutritional challenge. Proc. Natl Acad. Sci. USA 117, 29904–29913 (2020).
Sato, S. et al. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 34, 329–345 (2022).
Beyaz, S. et al. Dietary suppression of MHC class II expression in intestinal epithelial cells enhances intestinal tumorigenesis. Cell Stem Cell 28, 1922–1935 (2021).
Masri, S. et al. Lung adenocarcinoma distally rewires hepatic circadian homeostasis. Cell 165, 896–909 (2016).
Aiello, I. et al. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 6, eaaz4530 (2020).
Sulli, G. et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 553, 351–355 (2018).
Liu, S. et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports 2, 78–91 (2014).
Bocci, F. et al. Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc. Natl Acad. Sci. USA 116, 148–157 (2019).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Pein, M. et al. Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs. Nat. Commun. 11, 1494 (2020).
Dalerba, P. et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl Acad. Sci. USA 104, 10158–10163 (2007).
Pang, R. et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 6, 603–615 (2010).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Leung, C. et al. Lgr5 marks adult progenitor cells contributing to skeletal muscle regeneration and sarcoma formation. Cell Rep. 33, 108535 (2020).
Fatehullah, A. et al. A tumour-resident Lgr5+ stem-cell-like pool drives the establishment and progression of advanced gastric cancers. Nat. Cell Biol. 23, 1299–1313 (2021).
de Sousa e Melo, F. et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 543, 676–680 (2017).
Zhuang, J. et al. Cancer-associated fibroblast-derived miR-146a-5p generates a niche that promotes bladder cancer stemness and chemoresistance. Cancer Res. 83, 1611–1627 (2023).
Nallasamy, P. et al. Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1–CD44 axis. Gastroenterology 161, 1998–2013 (2021).
Yagita, K. et al. Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro. Proc. Natl Acad. Sci. USA 107, 3846–3851 (2010).
Matsu-Ura, T. et al. Intercellular coupling of the cell cycle and circadian clock in adult stem cell culture. Mol. Cell 64, 900–912 (2016).
Dierickx, P., Van Laake, L. W. & Geijsen, N. Circadian clocks: from stem cells to tissue homeostasis and regeneration. EMBO Rep. 19, 18–28 (2018).
Dierickx, P. et al. Circadian networks in human embryonic stem cell-derived cardiomyocytes. EMBO Rep. 18, 1199–1212 (2017).
Matsunaga, N. et al. Optimized dosing schedule based on circadian dynamics of mouse breast cancer stem cells improves the antitumor effects of aldehyde dehydrogenase inhibitor. Cancer Res. 78, 3698–3708 (2018).
Cao, H. et al. The Shh/Gli signaling cascade regulates myofibroblastic activation of lung-resident mesenchymal stem cells via the modulation of Wnt10a expression during pulmonary fibrogenesis. Lab. Invest. 100, 363–377 (2020).
Hwang-Verslues, W. W. et al. Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy. Proc. Natl Acad. Sci. USA 110, 12331–12336 (2013).
Colangelo, T. et al. Loss of circadian gene Timeless induces EMT and tumor progression in colorectal cancer via Zeb1-dependent mechanism. Cell Death Differ. 29, 1552–1568 (2022).
Papadaki, M. A. et al. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol. Cancer Ther. 18, 437–447 (2019).
Li, J., Sharkey, C. C., Wun, B., Liesveld, J. L. & King, M. R. Genetic engineering of platelets to neutralize circulating tumor cells. J. Control. Release 228, 38–47 (2016).
Harney, A. S. et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 5, 932–943 (2015).
Silver, A. C., Arjona, A., Hughes, M. E., Nitabach, M. N. & Fikrig, E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav. Immun. 26, 407–413 (2012).
Paiva, B. et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 122, 3591–3598 (2013).
Diamantopoulou, Z. et al. The metastatic spread of breast cancer accelerates during sleep. Nature 607, 156–162 (2022).
Matsumura, R. et al. The role of cell-autonomous circadian oscillation of Cry transcription in circadian rhythm generation. Cell Rep. 39, 110703 (2022).
Hartley, P. S. et al. Timed feeding of mice modulates light-entrained circadian rhythms of reticulated platelet abundance and plasma thrombopoietin and affects gene expression in megakaryocytes. Br. J. Haematol. 146, 185–192 (2009).
Wortzel, I., Dror, S., Kenific, C. M. & Lyden, D. Exosome-mediated metastasis: communication from a distance. Dev. Cell 49, 347–360 (2019).
Yeung, C. C. et al. Circadian regulation of protein cargo in extracellular vesicles. Sci. Adv. 8, eabc9061 (2022).
Khalyfa, A. et al. Exosomes and metabolic function in mice exposed to alternating dark–light cycles mimicking night shift work schedules. Front. Physiol. 8, 882 (2017).
Dong, P. et al. BMAL1 induces colorectal cancer metastasis by stimulating exosome secretion. Mol. Biol. Rep. 49, 373–384 (2022).
Ponert, J. M., Gockel, L. M., Henze, S. & Schlesinger, M. Unfractionated and low molecular weight heparin reduce platelet induced epithelial–mesenchymal transition in pancreatic and prostate cancer cells. Molecules 23, 2690 (2018).
Rodriguez-Martinez, A. et al. Exchange of cellular components between platelets and tumor cells: impact on tumor cells behavior. Theranostics 12, 2150–2161 (2022).
Ye, X. & Weinberg, R. A. Epithelial–mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol. 25, 675–686 (2015).
Lee, K. W. et al. PRRX1 is a master transcription factor of stromal fibroblasts for myofibroblastic lineage progression. Nat. Commun. 13, 2793 (2022).
Ocana, O. H. et al. Metastatic colonization requires the repression of the epithelial–mesenchymal transition inducer Prrx1. Cancer Cell 22, 709–724 (2012).
Du, B. et al. The transcription factor paired-related homeobox 1 (Prrx1) inhibits adipogenesis by activating transforming growth factor-β (TGFβ) signaling. J. Biol. Chem. 288, 3036–3047 (2013).
Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 111, 41–50 (2002).
Abbas, T. & Dutta, A. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer 9, 400–414 (2009).
Gaucher, J., Montellier, E. & Sassone-Corsi, P. Molecular cogs: interplay between circadian clock and cell cycle. Trends Cell Biol. 28, 368–379 (2018).
Fagiani, F. et al. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 7, 41 (2022).
Hernández-Camarero, P., López-Ruiz, E., Marchal, J. A. & Perán, M. Cancer: a mirrored room between tumor bulk and tumor microenvironment. J. Exp. Clin. Cancer Res. 40, 217 (2021).
Chen, P. et al. Circadian regulator CLOCK recruits immune-suppressive microglia into the GBM tumor microenvironment. Cancer Discov. 10, 371–381 (2020).
Xuan, W. et al. Circadian regulator CLOCK drives immunosuppression in glioblastoma. Cancer Immunol. Res. 10, 770–784 (2022).
Chan, A., Ma, S., Pearson, B. J. & Chan, D. Collagen IV differentially regulates planarian stem cell potency and lineage progression. Proc. Natl Acad. Sci. USA 118, e2021251118 (2021).
Shaashua, L. et al. Stromal expression of the core clock gene Period 2 is essential for tumor initiation and metastatic colonization. Front. Cell Dev. Biol. 8, 587697 (2020).
Panda, S. The arrival of circadian medicine. Nat. Rev. Endocrinol. 15, 67–69 (2019).
Sulli, G., Manoogian, E. N. C., Taub, P. R. & Panda, S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol. Sci. 39, 812–827 (2018).
Zhang, J., Lv, H., Ji, M., Wang, Z. & Wu, W. Low circadian clock genes expression in cancers: a meta-analysis of its association with clinicopathological features and prognosis. PLoS ONE 15, e0233508 (2020).
Cadenas, C. et al. Loss of circadian clock gene expression is associated with tumor progression in breast cancer. Cell Cycle 13, 3282–3291 (2014).
Brown, J. R. et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight 5, e133247 (2020).
Hirota, T. et al. Identification of small molecule activators of cryptochrome. Science 337, 1094–1097 (2012).
Miller, S. et al. CRY2 isoform selectivity of a circadian clock modulator with antiglioblastoma efficacy. Proc. Natl Acad. Sci. USA 119, e2203936119 (2022).
Dong, Z. et al. Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 9, 1556–1573 (2019).
Xia, L. et al. RORγt agonist enhances anti-PD-1 therapy by promoting monocyte-derived dendritic cells through CXCL10 in cancers. J. Exp. Clin. Cancer Res. 41, 155 (2022).
Mahalingam, D. et al. Phase 1 open-label, multicenter study of first-in-class RORγ agonist LYC-55716 (cintirorgon): safety, tolerability, and preliminary evidence of antitumor activity. Clin. Cancer Res. 25, 3508–3516 (2019).
Printezi, M. I. et al. Toxicity and efficacy of chronomodulated chemotherapy: a systematic review. Lancet Oncol. 23, e129–e143 (2022).
Lévi, F., Zidani, R. & Misset, J. L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350, 681–686 (1997).
von Roemeling, R. & Hrushesky, W. J. Circadian patterning of continuous floxuridine infusion reduces toxicity and allows higher dose intensity in patients with widespread cancer. J. Clin. Oncol. 7, 1710–1719 (1989).
Innominato, P. F., Karaboue, A., Bouchahda, M., Bjarnason, G. A. & Lévi, F. A. The future of precise cancer chronotherapeutics. Lancet Oncol. 23, e242 (2022).
Kroemer, G., Senovilla, L., Galluzzi, L., Andre, F. & Zitvogel, L. Natural and therapy-induced immunosurveillance in breast cancer. Nat. Med. 21, 1128–1138 (2015).
Cervantes-Silva, M. P. et al. The circadian clock influences T cell responses to vaccination by regulating dendritic cell antigen processing. Nat. Commun. 13, 7217 (2022).
Ince, L. M. et al. Influence of circadian clocks on adaptive immunity and vaccination responses. Nat. Commun. 14, 476 (2023).
Wang, C. et al. Dendritic cells direct circadian anti-tumour immune responses. Nature 614, 136–143 (2023).
Qian, D. C. et al. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 22, 1777–1786 (2021).
Karaboué, A. et al. Time-dependent efficacy of checkpoint inhibitor nivolumab: results from a pilot study in patients with metastatic non-small-cell lung cancer. Cancers 14, 896 (2022).
Kim, E. B. et al. Genome sequencing reveals insights into physiology and longevity of the naked mole rat. Nature 479, 223–227 (2011).
Chun, S. K. et al. Disruption of the circadian clock drives Apc loss of heterozygosity to accelerate colorectal cancer. Sci. Adv. 8, eabo2389 (2022).
Talib, W. H., Alsayed, A. R., Abuawad, A., Daoud, S. & Mahmod, A. I. Melatonin in cancer treatment: current knowledge and future opportunities. Molecules 26, 2506 (2021).
Mu, Q. & Najafi, M. Modulation of the tumor microenvironment (TME) by melatonin. Eur. J. Pharmacol. 907, 174365 (2021).
Van Dycke, K. C. et al. Chronically alternating light cycles increase breast cancer risk in mice. Curr. Biol. 25, 1932–1937 (2015).
Li, X. M., Claustrat, B., Hastings, M. H., Albrecht, U. & Lévi, F. [Interactions between clock gene mutation, circadian phenotype and tumor growth in mice]. Pathol. Biol. 55, 194–197 (2007).
Huber, A. L. et al. CRY2 and FBXL3 cooperatively degrade c-MYC. Mol. Cell 64, 774–789 (2016).
Ozturk, N., Lee, J. H., Gaddameedhi, S. & Sancar, A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc. Natl Acad. Sci. USA 106, 2841–2846 (2009).
Qu, M. et al. Circadian regulator BMAL1::CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc. Natl Acad. Sci. USA 120, e2214829120 (2023).
Acknowledgements
We thank D. Lane, Y. Zhang, J. Yeong and J. Wang for editing the manuscript. Our work was funded by the Agency for Science, Technology and Research (A*STAR) of Singapore and the Singapore Ministry of Health’s National Medical Research Council under OFIRG23jan-0037. H.G. is also supported by the Program of Shanghai Academic Research Leader (22XD1423400).
Author information
Authors and Affiliations
Contributions
Y.W. wrote the manuscript and made the figures. R.N., M.Q., N.S. and Y.X. conceived the structure of the manuscript and revised the manuscript. N.B., Y.X. and H.G. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cancer thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Narasimamurthy, R., Qu, M. et al. Circadian regulation of cancer stem cells and the tumor microenvironment during metastasis. Nat Cancer 5, 546–556 (2024). https://doi.org/10.1038/s43018-024-00759-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-024-00759-4