Abstract
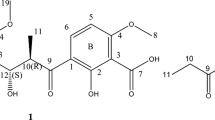
Chrysogeside B, a natural cerebroside, was efficiently synthesized from commercial feedstocks. The bioassays showed that compounds 4, 5 and 6 exhibited enhanced biological activities compared Chrysogeside B. Further studies revealed that free hydroxyl groups and glycosidic bond have significant impact on the antimicrobial activities. The synthesis of Chrysogeside B and analogues designed to allow identification of the features of this glycolipid required for recognition by tested bacteria and Hela cells is described.
Similar content being viewed by others
Introduction
Marine organisms have received widespread attention in the pharmaceutical industry due to the discovery of natural compounds with noteworthy biological activities1,2, cell growth regulation3,4, and potential utility for treatment of Alzheimer’s disease5,6,7, etc. In 2011, Peng’s group reported that marine-derived halotolerant fungal strain Penicillium chrysogenum could produce Chrysogeside B at 10% salinity that showed antimicrobial activity against Enterobacter aerogenes with an MIC value of 1.72 μM8 and cytotoxicity against Hela cells.
The importance of Chrysogeside B inspired us to explore the structure activity relationship. Specifically, we are interested to understand how the stereochemistry of glycosidic bond impacts the biological activities. We therefore conducted the enantioselective total synthesis of Chrysogeside B and some variants (Fig. 1). The biological activities were then assayed via growth inhibition studies against Enterobacter aerogenes, Hela cells and Escherichia coli.
There are generally two strategies for synthesis of cerebrosides. The first one contains the process in which an azide group is incorporated before generation of 1-glycosylated-2-azidosphingosine from the substituted glycosidic ligand, and then azide group is reduced to generates the amine for condensation with α-hydroxyl-β,γ-unsaturated acid9,10,11,12. The second approach, used by Wu13, Huang14, Lim15, and Thakur16, doesn’t rely on azide group to introduce amino group in sphingosine fragment synthesis.
Our route toward Chrysogeside B entails preparation of the three fragments: glycosidic ligand, sphingosine and α-hydroxyl-β,γ-unsaturated acid. Finally, ceramide is synthesized by combining activated α-hydroxyl-β,γ-unsaturated acid and protected sphingosine followed by glycosylation to produce Chrysogeside B17.
Results and Discussion
Total synthesis
Many syntheses of sphingosine and its analogues18,19,20,21 are based on serine or Garner aldehyde22,23,24,25,26. This chiral building block not only provides the C-2 stereocenter, but enables the introduction of the C-3 stereocenter upon addition of terminal alkynes to form fragment sphingosine22,23,24,25,26,27. We also employed the Garner aldehyde in our synthesis, as shown in Fig. 2. Initially, 2-hexyldihydrofuran 7 at very small amount was produced from dihydrofuran and t-BuLi at −78 °C followed by quenching with 1-iodohexane28. To scale up preparation of compound 7 at mild condition, we switched to n-BuLi at −78 °C, but found that reaction was too sluggish. After the addition of n-BuLi at −78 °C, however, the reaction mixture was warmed to 0 °C for 2 h and resulted in metallation of 2,3-dihydrofuran. Treatment of this solution with 1-iodohexane at −30 °C followed by warming to room temperature resulted in complete reaction after 12 h, as judged by TLC. After workup, the reaction product was subjected to a Kumada coupling9, providing (3E)-alcohol 8 in 80% yield over two steps, characteristic 1H NMR data of (3E)-alcohol 8, 1H NMR (CDCl3, 300 MHz) δ 5.10 (t, J = 7.9 Hz, 1H), 3.61 (t, J = 6.4 Hz, 2H), 2.27 (q, J = 6.8 Hz, 2H). The Appel reaction9 was employed to convert the hydroxyl of 8 to iodide (3E)-9 in 88% yield.
In the reaction of compound 9 with ethynyltrimethylsilane, the yield was very low at the beginning because plenty of byproduct terminal alkene formed from elimination of hydrogen iodide. We assumed that n-BuLi was not consumed completely during forming lithium salt of trimethylsilylethyne as described by Kenji Mori and Yuji Funaki29. Therefore, we tested fully forming lithium of ethynyltrimethylsilane by n-BuLi at −10 °C firstly, then adding compound 9 at −78 °C. Such a process could limit the byproduct below 10%. Subsequently, similar process was also employed for nucleophilic addition of Garner aldehyde with terminal alkyne to obtain the desired compound (4S,1′R,6′E)-16 as a single optical isomer by 1H NMR and optical rotation analysis with 83% yield, [α]D = −76.9 (c 0.56, CHCl3).
We next conducted reducing triple bond to double bond based on the study of Chaudhary Vinodand co-workers30, in which metal lithium in ethylamine was used as reductant, and cleanup was very complicated due to lithium is very hard to be accurately measured. Then, Red-Al31 was employed with 2.5 equiv to replace metal lithium. After reaction completed, 1 mL of saturated aqueous ammonium chloride was added, desired compound 17 was collected with 96% yield, [α]D = −23.1 (c 0.65, CHCl3), and characterized by 1H NMR, HRMS and 13C NMR. The hydroxyl group of compound 17 were protected with benzoyl chloride17 with 91% yield, and the isopropylidene was removed by amberlyst-1532 to get (2S,3R,4E,8E)-sphingosine 19 at yield of 74%, [α]D = −31.7 (c 0.87, CHCl3). (Refer supplementary information-pages 5–13).
Initially, the method of Murakami and co-workers17 was tried to synthesize compound 37 through forming glycosidic bond using tetrabenzoate α-D-Glucopyranosyl bromide 22 and 19 with catalyst AgOTf (Fig. 3). Unfortunately, the yield of product 37 was low, probably due to the fact that compound 19 was unreactive. Next we followed method of Pilgrim and Murphy33 to protect α-D-Glucose with benzoyl chloride to generate 21 ([α]D = +142.9 (c 0.55, CHCl3)). Bromination at C1 with hydrogen bromide furnished 2,3,4,6-tetra-O-benzoyl-α-D-glucopyranosyl bromide 22. Bromide 22 was hydrolyzed in the presence of silver carbonate to obtain 2,3,4,6-tetra-O-benzoyl-α-D-glucopyranose 23, [α]D = +111.4 (c 0.55, CHCl3). Compound 23 was treated with trichloroacetonitrile in the presence of DBU to generate 2,3,4,6-tetra-O-benzoyl-1-(2,2,2-trichloroethanimidate)-α-D-glucopyranoside 24 in 68% yield, [α]D = +95.7 (c 0.59, CHCl3). 2,3,4,6-Tetra-O-acetyl- 1-(2,2,2-trichloroethanimidate)-β-D-glucopyranoside 28 was obtained with the same process for compound 24 in 67% yield, [α]D = +7.9 (c 0.83, CHCl3) (Fig. 4). (Refer supplementary information-pages 13–17).
According to the method reported by Wu, Douglass and co-workers14, imidate 24 was combined with compound alcohol 19 in the presence of TMSOTf. Unfortunately, the glycosidic bond also was cleaved in the Boc deprotection with trifluoroacetic acid. Thus, synthetic pathway was modified to first synthesize ceramide followed by coupling of the ceramide with glycosidic ligand to form glycosidic bond.
To synthesize the α-hydroxyl-β,γ-unsaturated acid, terminal alkyne 30 was deprotonated with EtMgBr and added to diethyl oxalate. Selective reduction of α-keto-β,γ-acetylenic ester 31 by chiral borane34 provided enantiomerically enriched (2R)-32 characterized by 1H NMR, [α]D = −26.9 (c 0.54, CHCl3), 97% ee. Compound 32 was treated with HSi(OEt)Me2 and catalytic [Cp.Ru(MeCN)3]PF6 to generate trans addition product followed by removing dimethylethoxylsilyl group at low temperature in the presence of copper(I) iodide34,35 to obtain (2R,3E)-α-hydroxyl-β,γ-unsaturated ester 33 at yield of 70%, [α]D = −46.7 (c 0.55, CHCl3). Hydrolysis of the ester and acetylation of the alcohol were conducted. Activation of the acid with N-hydroxylsuccinimide17 furnished corresponding activated fatty acid ester (2R,3E)-36 (Fig. 5). (Refer supplementary information-pages 17–21).
According to reported methods13,17, (2S,3R,4E,8E)-sphingosine 20 from compound 19 reacted smoothly with compound 36 in the presence of DMAP to get ceramide 1, [α]D = +6.5 (c 0.70, CHCl3), with 65% yield (Fig. 6).
It has been noted in the literature that glycoside bond formation to synthesize cerebrosides from ceramide can lead to inversion of the glycosidic bond and epimerization at C230. These undesired isomerizations can be limited through optimization of reaction conditions17,36,37,38. Thus, we conducted a series of optimization experiments including solvents, temperature and catalyst loading, and found that when reactions were conducted under anhydrous conditions with diethyl ether/tetrahydrofuran (2:1, v/v) using 0.05 equiv TMSOTf as catalyst at −30 °C, no isomerization was found by NMR and the desired protected β-glucoside 2 was obtained with 60% yield, [α]D = +15.2 (c 1.14, CHCl3). Finally, sodium methoxide was used in the deprotection, resulting in the target product Chrysogeside B (3) in 85% yield, characterized by NMR spectra and [α]D = −8.1 (c 0.5, CH3OH) agreed well with lit.8 [α]D = −8.0 (c 0.5, CH3OH) (Fig. 7). Compound 4 was synthesized with the same process for compound 2 from acetylated glycosyl donor 28 in 50% yield, and compounds 5 and 6 were prepared as the same as the process for Chrysogeside B with 80% and 89% yield. (Refer supplementary information-pages 21–25).
Antimicrobial activities and cytotoxic assays
According to the report of Peng’s group8, the antimicrobial activities against Enterobacter aerogenes were evaluated by an agar dilution method (Fig. 8) (refer supplementary information-pages 67–71). As the results showed, the antimicrobial activities of compounds 1, 2, 4–6 were better than Chrysogeside B at 100 and 1,000 μM. The antimicrobial activities of analogues 1, 2, 6 were also better than Chrysogeside B at 5 and 10 μM, their MIC were less than 5 μM, the corresponding ceramide elicited better antimicrobial activity than Chrysogeside B. Replacing β-glycosidic bond in Chrysogeside B with α-glycosidic bond, the diastereoisomer of Chrysogeside B at higher concentrations displayed higher antimicrobial activities.
(A,B) The antimicrobial activities with synthetic compounds 1–6 against Enterobacter aerogenes and Escherichia coli. Incubation after 24 h, and zones of inhibition (mm in diameter) were recorded. (C) The cytotoxic assays against Hela cells with synthetic compounds 1–6 at different concentrations by the MTT method. Data are expressed as means ± SD of the inhibition rate of Hela cells by synthetic compounds 1–6 at 100, 500 μM. *P < 0.01 vs control.
This result suggests that the glycosidic bond of chrysogesides exerts a greater influence on antimicrobial activities, and the fully protected compounds 2 and 4 also have weak antibacterial activity. The same result is shown in the antimicrobial activities of Escherichia coli and cytotoxic assays, especially at 100 μM (Fig. 8). Compounds 5, 6 showed antimicrobial activities against Escherichia coli with MIC less than 5 μM, and cytotoxic effects against Hela cells with IC50 less than 100 μM.
Conclusion
In conclusion, we presented a convergent synthetic approach to Chrysogeside B and five of its analogues based on the use of two chiral building blocks prepared by means of catalytic diastereoselective reactions. Based on results from assays, it was found that the free hydroxyl groups and glycosidic bond have significant impact on antimicrobial activities and cytotoxicities of cerebrosides and ceramide against Enterobacter aerogenes and Hela cells. These results are very helpful for optimizing glycolipid structures for Enterobacter aerogenes inhibitors.
Methods
General Information
1H NMR and 13C NMR spectra were recorded on a Bruker Avance DPX 300 MHz instrument (Bruker, Billerica, MA 01821-3991, USA), TMS as the internal standard. 1H NMR data are reported as follows: chemical shift, multiplicity (s = singlet; d = doublet; q = quartet; m = multiplet; br = broad), coupling constant (Hz), and integral. Data for 13C NMR spectra are reported in terms of chemical shift. Mass spectrometric data were obtained on Agilent Accurate-Mass-Q-TOF MS 6520 system equipped with an Electrospray ionization (ESI) source (Agilent, Santa Clara, CA 95051, USA). Specific rotations were obtained on a High Accurary Polarimeter Rudolph Autopl VI (Rudolph, Wilmington, Massachusetts 01887, USA). Toluene and DCM were freshly distilled after dried by calcium hydride under nitrogen, diethyl ether and THF were freshly distilled after dried by Lithium aluminum hydride. Unless otherwise stated, all reagents were commercially available and were used without purification. Organic solutions were concentrated under reduced pressure on a rotary evaporator or an oil pump. Reactions were monitored through thin layer chromatography (TLC) on silica gel-precoated glass plates (0.25 mm thickness, SiliCycle silica gel). Flash column chromatography was performed using Qingdao Haiyang flash silica gel (200–300 mesh).
(2R,3E)-2-acetoxy-N-[(2S,3R,4E,8E)-1-hydroxy-3-benzoyloxy-9-methylpentadec-4,8-dien-2-yl]nonadec-3-enamide 1
Triethylamine (0.15 mL, 1.50 mmol) was added to a solution of compound 20 (447 mg, 1.20 mmol), compound 36 (541 mg, 1.20 mmol), DCM (30 mL) and 4-dimethylaminopyridine (10 mg) at room temperature, and the reaction mixture was stirred for overnight. After the reaction was completed by TLC detection, the solution was concentrated under vacuum. The residue was purified using silica gel chromatography (25% ethyl acetate in hexanes) to give compound 1 as a colorless amorphous solid 552 mg, yield: 65%. Analytical data for 1: [α]D = +6.5 (c0.70, CHCl3); 1H NMR (300 MHz,CDCl3) δ 8.05 (d, J = 7.9 Hz, 2H, Ar-H), 7.61 (t, J = 6.8 Hz, 1H, Ar-H), 7.48 (t, J = 7.6 Hz, 2H, Ar-H), 6.79 (d, J = 8.4 Hz, 1H, C=ONH), 6.03–5.00 (m, 7H, CH=CH, OAcCH, OBzCH), 4.33–4.13 (m, 1H, NHCH), 3.83–3.62 (m, 2H, CH2O), 2.26–1.56 (m, 11H, OAc, CH=CHCH2), 1.49 (s, 3H, CH=CHCH3), 1.42–1.14 (m, 34H, CH2), 0.87 (t, J = 6.6 Hz, 6H, CH2CH3); 13C NMR (75 MHz, CDCl3) δ169.4, 169.1, 166.7, 138.2, 137.3, 137.1, 133.7, 131.0, 123.0, 129.8, 128.7, 126.2, 125.5, 124.9, 123.1, 74.8, 74.7, 61.7, 54.0, 39.8, 32.7, 32.5, 32.1, 31.8, 30.3, 29.9, 29.8, 29.6, 29.5, 29.4, 29.3, 29.1, 28.9, 28.1, 28.0, 27.4, 27.3, 26.9, 22.8, 21.1, 16.2, 16.0, 14.2; HRMS (ESI): m/z [M+Na]+ calcd for C44H71NNaO6: 732.5174; found: 732.5178.
(2R,3E)-2-acetoxy-N-[(2S,3R,4E,8E)-1-(2,3,4,6-tetrabenzoyloxy-l-β-D-glucopyranosyloxy)-3-benzoyloxy-9-methylpentadec-4,8-dien-2-yl]nonadec-3-enamide 2
Mixed solvents (anhydrous ethyl ether/THF = 2:1, 5 mL) was added to a solution of compound 1 (100 mg, 0.14 mmol), compound 24 (121 mg, 0.15 mmol), 4A molecular sieve (1 g) under the protection of nitrogen. The mixed solution was stirred at room temperature for 1 h, and was cooled to −30 °C, and then trimethylsilyl trifluoromethanesulfonate (0.65 uL) was dropped. After the reaction was completed by TLC detection, triethylamine (1 mL) was added to the solution. The mixture was filtered and the filtrate was concentrated under vacuum. The residue was purified using silica gel chromatography (20% ethyl acetate in hexanes) to give compound 2 as a colorless amorphous solid 108 mg, yield: 60%. Analytical data for 2: [α]D = +15.2 (c1.14, CHCl3); 1H NMR (300 MHz,CDCl3) δ 8.10 (d, J = 7.2 Hz, 2H, Ar-H), 8.00–7.81 (m, 6H, Ar-H), 7.72–7.30 (m, 17H, Ar-H), 6.44 (d, J = 9.2 Hz, 1H, C=ONH), 6.02–5.84 (m, 2H, BzOCHCH=CH, AcOCHCH=CH), 5.84–5.44 (m, 6H, CH2CH=CH, H-3, H-4, H-2), 6.02–5.21 (m, 2H, OAcCH, OBzCH), 4.79 (d, J = 8.0 Hz, 1H, H-1), 4.56–4.25 (m, 3H, NHCH, H-6), 4.12–4.00 (m, 1H, H-5), 3.69–3.56 (m, 1H, CHaHbO), 3.41–3.26 (m, 1H, CHaHbO), 2.10 (s, 3H, OAc), 2.04–1.65 (m, 8H, CH=CHCH2), 1.45 (s, 3H, CH=CHCH3), 1.39–1.15 (m, 34H, CH2), 0.88 (t, J = 6.1 Hz, 6H, CH2CH3); 13C NMR (75 MHz, CDCl3) δ69.4, 168.6, 166.1, 165.9, 165.3, 138.0, 136.1, 133.7, 133.6, 133.5, 133.4, 133.2, 133.1, 130.1, 123.0, 129.9, 129.8, 129.6, 129.5, 129.3, 129.2, 129.1, 128.9, 128.8, 128.7, 128.6, 128.5, 125.6, 122.9, 101.0, 72.4, 72.3, 69.7, 63.1, 50.9, 38.0, 32.4, 32.1, 31.7, 29.8, 29.6, 29.5, 29.4, 28.8, 27.1, 23.5, 22.8, 22.7, 20.8, 14.2, 14.1; HRMS (ESI): m/z [M+Na]+ calcd for C78H97NNaO15: 1310.6749; found: 1310.6742.
Chrysogeside B (3)
Sodium methoxide solution (0.05 mL 0.5 M in methanol, 0.025 mmol) was added to the solution of compound 2 (90 mg, 0.09 mmol) and anhydrous methanol (5 mL) at 0 °C, and the solution was stirred at room temperature for 2 h. After the reaction was completed by TLC detection, ambrest 15 was added to adjust pH 6–7. After the mixture was filtered and filtrate was concentrated under vacuum. The residue was purified using silica gel chromatography (10% methanol /acetate in chloroform) to give compound 3 as a colorless amorphous solid 43 mg, yield: 85%. Analytical data for 3: [α]D = −8.1 (c 0.5, CH3OH); (lit.9 [α]D = −8.0 (c 0.5, CH3OH)); 1H NMR (300 MHz, CD3OD) δ 5.96–5.80 (m, 1H, HOCHCH=CH), 5.80–5.67 (m, 1H, HOCHCH=CH), 5.66–5.26 (m, 3H, CH2CH=CH), 4.63–4.39 (m, 1H, O=CCHOH), 4.27 (d, J = 7.6 Hz, 1H, H-1), 4.23–4.05 (m, 3H, COCH2, NHCHCHOH), 4.04–3.92 (m, 1H, NHCHCHOH), 3.92–3.81 (m, 1H, H-3), 3.80–3.60 (m, 2H, H-4, H-2), 3.30–3.26 (m, 2H, H-6), 3.25–3.15 (m, 1H, H-5), 2.36–1.89 (m, 6H, CH=CHCH2), 1.73–1.48 (m, 2H, CH2(CH2)4CH3), 1.43 (s, 3H, CH=CHCH3), 1.29 (s, 34H, CH2), 0.90 (t, J = 6.6 Hz, 6H, CH2CH3); 13C NMR (75 MHz, CD3OD) δ 175.5, 135.6, 135.1, 134.8, 132.4, 130.9, 129.9, 129.0, 104.7, 78.0, 75.0, 74.1, 73.3, 72.9, 71.6, 69.6, 62.7, 55.0, 42.8, 34.0, 33.4, 33.1, 31.2, 30.8, 30.7, 30.4, 30.2, 26.9, 25.0, 24.6, 23.7, 19.5, 14.4; HRMS (ESI): m/z [M+Na]+ calcd for C41H75NNaO9: 748.5334; found: 748.5338.
(2R,3E)-2-Acetoxy-N-[(2S,3R,4E,8E)-1-(2,3,4,6-tetraacetyloxy-l-α-D-glucopyranosyloxy)-3-benzoyloxy-9-methylpentadec-4,8-dien-2-yl]nonadec-3-enamide 4
With the same process for the synthesis of 2, the compound 4 was obtained from compound 28 and compound 1 as a colorless amorphous solid 92 mg, yield: 50%. Analytical data for 4: [α]D = +19.3 (c 0.76, CHCl3); 1H NMR (300 MHz, CDCl3) δ 8.02 (d, J = 7.4 Hz, 2H, Ar-H), 7.57 (t, J = 7.2 Hz, 1H, Ar-H), 7.44 (t, J = 7.4 Hz, 2H, Ar-H), 6.47 (d, J = 9.2 Hz, 1H, C=ONH), 6.03–5.73 (m, 2H, BzOCHCH=CH, AcOCHCH=CH), 5.73–5.41 (m, 5H, CH2CH=CH, H-3, H-4), 5.41–5.02 (m, 2H, OAcCH, OBzCH), 4.99 (d, J = 3.6 Hz, 1H, H-1), 4.50–4.36 (m, 1H, H-2), 3.86–3.72 (m, 2H, H-5, H-6a), 4.19–4.01 (m, 2H,H-6b, NHCH), 3.86–3.72 (m, 1H CHaHbO), 3.66–3.45 (m, 1H, CHaHbO), 2.18–2.01 (m, 15H, OAc), 1.99–1.60 (m, 8H, CH=CHCH2), 1.47 (s, 3H, CH=CHCH3), 1.44–1.11 (m, 34H, CH2), 0.87 (t, J = 5.6 Hz, 6H CH2CH3); 13C NMR (75 MHz, CDCl3) δ 169.6, 169.5, 169.2, 168.5, 168.4, 168.3, 168.0, 137.1, 135.0, 132.6, 132.2, 128.8, 128.7, 127.6, 127.4, 124.5, 121.9, 95.9, 73.7, 73.6, 73.4, 71.5, 70.9, 67.3, 60.8, 60.4, 52.8, 36.9, 32.9, 31.3, 30.9, 30.6, 28.7, 28.6, 28.5, 28.3, 27.7, 27.6, 24.6, 23.9, 22.3, 21.7, 21.5, 19.8, 19.5, 18.2, 13.1, 13.0; HRMS (ESI): m/z [M+Na]+ calcd for C58H89NNaO15: 1062.6114; found: 1062.6107.
(2R,3E)-2-Hydroxy-N-[(2S,3R,4E,8E)-l-α-D-glucopyranosyloxy-3-hydroxy-9-methylpentadec-4,8-dien-2-yl]nonadec-3-enamide 5
With the same process for the synthesis of 3, the compound 5 was obtained from compound 4 and sodium methoxide solution (0.5 M in methanol) as a colorless amorphous solid 41 mg, yield: 80%. Analytical data for 5: [α]D = +5.4 (c 0.50, CH3OH); 1H NMR (300 MHz, CD3OD) δ 5.96–5.81 (m, 1H, HOCHCH=CH), 5.80–5.67 (m, 1H, HOCHCH=CH), 5.66–5.22 (m, 3H, CH2CH=CH), 4.72–4.44 (m, 2H, O=CCHOH, H-1), 4.32–4.06 (m, 3H, COCH2, NHCHCHOH), 4.06–3.93 (m, 1H, NHCHCHOH), 3.94–3.80 (m, 1H, H-3), 3.80–3.48 (m, 3H, H-4, H-2, H-5), 3.30–3.14 (m, 2H, H-6), 2.36–1.47 (m, 8H, CH=CCH2, CH=CHCH2), 1.45 (s, 3H, CH=CHCH3), 1.41–1.23 (m, 34H, CH2), 0.92 (t, J = 6.7 Hz, 6H, CH2CH3); 13C NMR (75 MHz, CD3OD) δ 175.6, 135.4, 134.6, 134.3, 100.3, 129.0, 103.3, 78.1, 76.2, 75.8, 74.1, 73.3, 71.8, 62.0, 56.4, 42.3, 34.0, 33.4, 33.0, 31.2, 30.8, 30.7, 30.6, 30.4, 30.2, 26.9, 25.0, 24.6, 23.7, 20.9, 14.4; HRMS (ESI): m/z [M+Na]+ calcd for C41H75NNaO9: 748.5334; found: 748.5335.
(2R,3E)-2-Hydroxy-N-[(2S,3R,4E,8E)-1-hydroxy-3-hydroxy-9-methylpentadec-4,8-dien-2-yl]nonadec-3-enamide 6
With the same process for the synthesis of 3, the compound 6 was obtained from compound 1 and sodium methoxide solution (0.5 M in methanol) as a colorless amorphous solid 71 mg, yield: 89%. Analytical data for 6: [α]D = −6.2 (c 0.65, CH3OH); 1H NMR (300 MHz,CDCl3) δ 6.09–5.67 (m, 2H, HOCHCH=CH), 6.67–5.03 (m, 3H, CH2CH=CH), 4.72–4.14 (m, 3H, CHNH, CHOH), 3.99–3.39 (m, 2H, CH2O), 2.35–1.49 (m, 8H, CH=CHCH2), 1.48–0.93 (m, 37H, CH=CHCH3,CH2), 0.88 (t, J = 5.8 Hz, 6H, CH3); 13C NMR (75 MHz, CD3OD) δ 175.7, 135.7, 134.7, 130.9, 129.0, 128.5, 127.7, 74.0, 73.2, 62.0, 56.6, 42.2, 34.0, 33.4, 33.0, 31.2, 30.8, 30.7, 30.6, 30.4, 30.3, 30.2, 28.9, 26.9, 25.0, 23.7, 14.4; HRMS (ESI): m/z [M+H]+ calcd for C35H66NO4: 564.4991; found: 564.4997.
Bioassay Protocols
Antimicrobial Assays
The antimicrobial activities against Enterobacter aerogenes (ATCC51697) and Escherichia coli (ATCC13048) were evaluated by an agar dilution method. The tested strains were cultivated in Nutrient agar plates and Luria-Bertani agar plates for bacteria at 37 °C. Compounds 1–6 and positive controls were dissolved in methanol at different concentrations from 1,000 to 0.1 μM by the continuous 10-fold dilution methods and 2-fold dilution methods. A 5 μL quantity of test solution was absorbed by a paper disk (6 mm diameter) and placed on the assay plates. After 24 h incubation, zones of inhibition (mm in diameter) were recorded. Ciprofloxacin (5 μg/disk), and Gentamicin (10 μg/disk) and methanol (5 μL/disk) were used as positive control and blank control for Enterobacter aerogenes and Escherichia coli with zones of inhibition (mm in diameter) of 26.5, 24.0, 6.0, and 28.5, 21.5, 6.0 mm, respectively.
Cytotoxic Assays
Cytotoxicity was assayed by the MTT methods. Hela cells line was grown in DMEM supplemented with 10% FBS under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Cell suspension (100 μL, a density of 5 × 104 cell mL−1) was plated in 96-well microtiter plates and incubated for 24 h. Then, 100 μL of the test solutions (in DMEM), which was at different concentrations between 500 and 100 μM by the dilution methods, were added to each well and further incubated for 72 h. The MTT solution (20 μL, 5 mg/mL in IPMI-1640 medium) was then added to each well and incubated for 4 h. Old medium containing MTT (150 μL) was then gently replaced by DMSO, and shaking was conducted to dissolve completely formazan crystals formed. Absorbance was then determined on a Spectra Max Plus plate reader at 570 nm.
Additional Information
How to cite this article: Liu, R. et al. Synthesis of Chrysogeside B from Halotolerant Fungus Penicillium and Its Antimicrobial Activities Evaluation. Sci. Rep. 7, 45927; doi: 10.1038/srep45927 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Duan, J. J. et al. Dietary cerebroside from sea cucumber (Stichopus japonicus): absorption and effects on skin barrier and cecal short-chain fatty acids. J. Agric. Food Chem. 64, 7014–7021 (2016).
Bourbon, N. A., Yun, J. & Kester, M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J. Bio. Chem. 275(45), 35617–35623 (2000).
Tao, W. W. et al. Two new cerebrosides from the pollen of Typha angustifolia . Fitoterapia. 81(3), 196–199 (2010).
Liu, P., Liu, L., Tang, Y. P., Duan, J. A. & Yang, N. Y. A new cerebroside and its anti-proliferation effect on VSMCs from the radix of Cyperus rotundus L. Chin. Chem. Lett. 21(5), 606–609 (2010).
Assali, M., Cid, J. J., Fernandez, I. & Khiar, N. Supramolecular diversity through click chemistry: switching from nanomicelles to 1D-nanotubes and tridimensional hydrogels. Chem. Mater. 25(21), 4250–4261 (2013).
Ahmad, N. et al. Influence of nonionic branched-chain alkyl glycosides on a model nano-emulsion for drug delivery systems. Colloisds Surf., B. 115, 267–274 (2014).
Kowa, T. K. et al. Antiplasmodial activity and cytotoxicity of isolated compound from the stem bark of Anthocleista liebrechtsiana . Rec. Nat. Prod. 10(3), 287–293 (2016).
Peng, X. P. et al. Cerebrosides and 2-pyridone alkaloids from the halotolerant fungus Penicillium chrysogenum grown in a hypersaline medium. J. Nat. Prod. 74(5), 1298–1302 (2011).
Black, F. J. & Kocienski, P. J. Synthesis of phalluside-1 and Sch II using 1,2-metallate rearrangements. Org. Biomol. Chem. 8(5), 1188–1193 (2010).
Sawant, R. C. et al. Synthesis of hydroxylated analogues of α-galactosyl ceramide (KRN7000) with varying stereochemistry. Eur. J. Org. Chem. 33, 7611–7623 (2013).
Pilgrim, W., O’Reilly, C. & Murphy, P. V. Synthesis of α-O- and α-S-glycosphingolipids related to Sphingomonous cell wall antigens using anomerization. Molecules. 18(9), 11198–11218 (2013).
Long, D. E., Karmakar, P., Wall, K. A. & Sucheck, S. J. Synthesis of α-L-rhamnosyl ceramide and evaluation of its binding with anti-rhamnose antibodies. Bioorg. Med. Chem. 22(19), 5279–5289 (2014).
Wu, D. et al. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. Natl. Acad. Sci. USA 102(5), 1351–1356 (2005).
Huang, W. F., Li, Q. R., Chao, L. M., Lei, X. S. & Wei, B. G. Asymmetric synthesis of ceramide sphingolipid based on (2S,3S,4S)-3,4-dihydroxy-5-(hydroxymethyl) pyrrolidine lactam. Tetrahedron Lett. 51(33), 4317–4319 (2010).
Lim, C. et al. Design and evaluation of ω-hydroxy fatty acids containing α-GalCer analogues for CD1d-mediated NKT cell activation. ACS Med. Chem. Lett. 5(4), 331–335 (2014).
Thakur, M. S., Khurana, A., Kronenberg, M. & Howell, A. R. Synthesis of a 2″-deoxy-β-GalCer. Molecules. 19(7), 10090–10102, 10113 (2014).
Teiichi, M., Reiko, H. & Kiyotaka, F. Efficient stereocontrolled synthesis of sphingadienine derivatives. Tetrahedron. 61(39), 9233–9241 (2005).
Hino, T., Nakakyama, K., Taniguchi, M. & Nakagawa, M. A new to racemic erythro-sphingosine and ceramides. The 1,2- versus 1,4-addition reaction of hexadec-2-enal with 2-nitroethanol. J. Chem. Soc. 9, 1687–1690 (1986).
Obayashi, M. & Schlosser, M. An efficient synthesis of (2S,3R)- and (2S,3S)-sphingosine. Chem. Lett. 11, 1715–1718 (1985).
Chaundhari, V. D., Ajish, K. K. & Dhavale, D. D. An efficient synthesis of D-erythro- and D-threo-sphingosine from D-glucose: olefin cross-metathesis approach. Org. Lett. 7(26), 5805–5807 (2005).
Johnson, D. V., Felfer, U. & Griengl, H. A chemoenzymatic access to D- and L-sphingosines employing hydroxynitrile lyases. Tetrahedron. 56(5), 781–790 (2000).
Garner, P. Stereocontrolled addition to a penaldic acid equivalent: an asymmetric synthesis of threo-β-hydroxy-L-glutamic acid. Tetrahedron Lett. 25(51), 5855–5858 (1984).
Garner, P. & Ramakanth, S. Stereodivergent synthesis of threo and erythro 6-amino-6-deoxyheptosulose derivatives via an optically active oxazolidine aldehyde. J. Org. Chem. 51(13), 2609–2612 (1986).
Garner, P. & Park, J. M. The synthesis and configurational stability of differentially protected β-hydroxy-α-amino aldehydes. J. Org. Chem. 52(12), 2361–2364 (1987).
Garner, P. & Park, J. M. Asymmetric synthesis of 5-O-carbamoylpolyoxamic acid from D-serine. J. Org. Chem. 53(13), 2979–2984 (1988).
Garner, P., Park, J. M. & Malecki, E. A stereodivergent synthesis of D-erythro-sphingosine and D-threo-sphingosine from L-serine. J. Org. Chem. 53(18), 4395–4398 (1988).
Yamanoi, T. et al. Horner-Wittig reaction of dimethyl 2,3-O-isopropylidene-D-glyceroylmethylphosphonate and its application to the formal synthesis of D-erythro-C18-sphingosine. Chem. Lett. 2, 335–336 (1989).
Simon, N. K., Markus, N. & Hermann, A. W. Bidentate lewis acids for the activation of 1,2-diazines-anew mode of catalysis. Eur. J. Org. Chem. 17, 3238–3245 (2011).
Mori, K. & Funaki, Y. Synthesis of sphingosine relatives. III. Synthesis of (4E,8E,2S,3R,2′R)-N-2′-hydroxyhexadecanoyl-1-O-β-D-glucopyranosyl-9-methyl-4,8-sphingadienine, the fruiting-inducing cerebroside in a basidiomycete Schizophyllum commune. Tetrahedron. 41(12), 2379–2386 (1985).
Vinod, C. et al. Synthesis of fungal glycolipid asperamide B and investigation of its ability to stimulate natural killer T cells. Org. Lett. 15(20), 5242–5245 (2013).
Lu, X. Q. & Robert, B. Synthesis of a photoactivatable (2S,3R)-sphingosylphosphorylcholine analogue. J. Org. Chem. 70(12), 4746–4750 (2005).
De Jonghe, S. et al. Synthesis of fluorinated sphinganine and dihydroceramide analogues. Eur. J. Org. Chem. 18, 3177–3183 (2000).
Wayne, P. & Paul, V. M. SnCl4- and TiCl4-catalyzed anomerization of acylated O- and S-glycosides: analysis of factors that lead to higher α:β anomer ratios and reaction rates. J. Org. Chem. 75(20), 6747–6755 (2010).
Prévost, Sébastien, Ayad, Tahar, Phansavath, Phannarath & Ratovelomanana-Vidal, Virginie . Total synthesis of symbioramide: a flexible approach for the efficient preparation of structural isomers. Adv. Synth. Catal. 353(17), 3213–3226 (2011).
Wang, L. et al. Catalytic enantioselective synthesis of optically active α-hydroxyl-β,γ-unsaturated acid esters as novel side chains of cerebrosides. Tetrahedron: Asymmetry. 24, 173–177 (2013).
Martin, A. et al. Catching elusive glycosyl cations in a condensedphase with HF/SbF5 superacid. Nature Chem. 8(2), 186–191 (2015).
Zhu, X. M. & Schmidt, R. R. New principles for glycoside-bond formation. Angew. Chem. Int. Ed. 48, 1900–1934 (2009).
Chen, X. S., Wu, Y. L. & Chen, D. H. Structure determination and synthesis of a new cerebroside isolated from the traditional Chinese medicine Typhonium giganteum Engl. Tetrahedron Lett. 43(19), 3529–3532 (2002).
Acknowledgements
We gratefully acknowledge the financial support by the National Natural Science Foundation of China (NNSFC) (No. 21172256), the National Basic Research Program of China (No. 2010CB126104). Also, we thank the personnel of Prof. Shiyan Qiao and Assoc. Prof. Xiangfang Zeng’s group, College of Animal Science and Technology, China Agricultural University, for antimicrobial activities and cytotoxic assays, and thank Prof. Patrick J. Walsh in University of Pennsylvania for his help during manuscript preparation.
Author information
Authors and Affiliations
Contributions
R.Q. Liu, L. Wang and S.Z. Liu conceived and designed the project. R.Q. Liu, Q.B. Li, M. Liao, Z.K. Yang, Y. Huang and C. Lv conducted the experiments. R.Q. Liu, B. Zheng, J.C. Zhong, Q.H. Bian, M. Wang and S.Z. Liu analyzed the data and prepared the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Liu, R., Wang, L., Li, Q. et al. Synthesis of Chrysogeside B from Halotolerant Fungus Penicillium and Its Antimicrobial Activities Evaluation. Sci Rep 7, 45927 (2017). https://doi.org/10.1038/srep45927
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45927
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.