Abstract
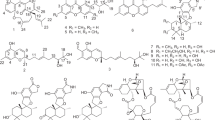
Three new compounds, including one steroid named penivariod A (1) and two polyketides penivarides A and B (2 and 3), as well as six known derivatives (4–9) were isolated from Penicillium variabile EN-394, a fungus afforded from the marine red alga Rhodomela confervoides. Their structures were elucidated by analysis of the HRESIMS, 1D and 2D NMR. The absolute stereochemistry was determined by X-ray crystallographic data, gauge-independent atomic orbital (GIAO) NMR shift calculation followed by DP4+ analysis combined with calculated electronic circular dichroism (ECD). Antimicrobial activities for the new compounds (1–3) were evaluated against human- and aquatic-pathogenic bacteria as well as plant pathogenic fungi. Compound 1 exhibited potent antimicrobial activity against most of the pathogenic strains, especially for Escherichia coli and Pseudomonas aeruginosa, with MIC values of 1.0 and 2.0 μg ml−1, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Yilmaz N, et al. Taxonomic re-evaluation of species in Talaromyces section Islandici, using a polyphasic approach. Persoonia 2016;36:37–56.
Dong YS, et al. Cathepsin B inhibitory tetraene lactones from the fungus Talaromyces wortmannii. Helv Chim Acta. 2009;92:567–74.
Benjamin CR. Ascocarps of Aspergillus and Penicillium. Mycologia 1955;47:669–87.
Petruccioli M, Federici F. Glucose oxidase production by Penicillium variabile P16: effect of medium composition. J Appl Microbiol. 1993;75:369–72.
He XQ, et al. Varilactones and wortmannilactones produced by Penicillium variabile cultured with histone deacetylase inhibitor. Arch Pharm Res. 2018;41:57–63.
Dong YS, et al. Wortmannilactones A–D, 22-membered triene macrolides from Talaromyces wortmannii. J Nat Prod. 2006;69:128–30.
He XQ, et al. Varitatin A, a highly modified fatty acid amide from Penicillium variabile cultured with a DNA methyltransferase inhibitor. J Nat Prod. 2015;78:2841–5.
Zhelifonova VP, Antipova TV, Ozerskaya SM, Ivanushkina NE, Kozlovskii AG. The fungus Penicillium variabile Sopp 1912 isolated from permafrost deposits as a producer of rugulovasines. Microbiology 2006;75:644–8.
Shao YT, et al. New azaphilones from Penicillium variabile, a fungal endophyte from roots of Aconitum vilmorinianum. J Antibiot. 2020;73:77–81.
Abe M. Rugulovasine: U. S. Patent No. 3,651,220. Washington, DC: U. S. Patent and Trademark Office; 1972.
Liu H, et al. Chermesins A–D: meroterpenoids with a drimane-type spirosesquiterpene skeleton from the marine algal-derived endophytic fungus Penicillium chermesinum EN-480. J Nat Prod. 2016;79:806–11.
Zhang FZ, Li XM, Yang SQ, Meng LH, Wang BG. Thiocladospolides A−D, 12-membered macrolides from the mangrove-derived endophytic fungus Cladosporium cladosporioides MA-299 and structure revision of pandangolide 3. J Nat Prod. 2019;82:1535–41.
Yang SQ, et al. Antibacterial anthraquinone derivatives isolated from a mangrove-derived endophytic fungus Aspergillus nidulans by ethanol stress strategy. J Antibiot. 2018;71:778–84.
Li HL, et al. Induced terreins production from marine red algal-derived endophytic fungus Aspergillus terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531. J Antibiot. 2020;73:108–11.
Cui CM, et al. 7-Nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspergillus ochraceus EN-31. J Nat Prod. 2010;73:1780–4.
D’Auria MV, et al. Unique 3β-O-methylsterols from the pacific sponge Jereicopsis Graphidiophora. J Nat Prod. 1992;55:311–20.
Matsumori N, Kaneno D, Murata M, Nakamura H, Tachibana K. Stereochemical determination of acyclic structures based on carbon-proton spin-coupling constants. A method of configuration analysis for natural products. J Org Chem. 1999;64:866–76.
Barrero AF, et al. Oxygenated diterpenes and other constituents from Moroccan Juniperus phoenicea and Juniperus thurifera var. africana. Phytochemistry 2004;65:2507–15.
Sims JJ, Pettus JA. Isolation of free cis and trans-phytol from the red alga Gracilaria Andersoniana. Phytochemistry 1976;15:1076–7.
Kim CS, et al. Structural characterization of terpenoids from Abies holophylla using computational and statistical methods and their biological activities. J Nat Prod. 2018;81:1795–802.
Grimblat N, Zanardi MM, Sarotti AM. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J Org Chem. 2015;8:12526–34.
Smith SG, Goodman JM. Assigning Stereochemistry to Single Diastereoisomers by GIAO NMR Calculation: The DP4 Probability. J Am Chem Soc. 2010;132:12946–59.
Lu T, Chen FW. Multiwfn: A multifunctional wavefunction analyzer. J Comput Chem. 2012;33:580–92.
Crystallographic data of compound 1 have been deposited in the Cambridge Crystallographic Data Centre as CCDC 1906035. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/ data request/cif (or from the CCDC, 12 Union Road, Cambridge CB21EZ, U.K.; fax: +44-1223-336-033; e-mail: deposit@ccdc.cam.ac.uk).
Sheldrick GM. SADABS, Software for empirical absorption correction. Germany: University of Gottingen; 1996.
Sheldrick GM. SHELXTL, Structure determination software programs. Madison, WI: Bruker Analytical X-ray System Inc.; 1997.
Sheldrick GM. SHELXL-97 and SHELXS-97, program for X-ray crystal structure solution and refinement. Germany: University of Gottingen; 1997.
Gaussian 16, Revision C.01, Frisch MJ, et al. Gaussian, Inc. 340 Quinnipiac St., Bldg. 40, Wallingford CT 06492, 2019.
Pierce CG, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3:1494–500.
Acknowledgements
Financial support from the National Natural Science Foundation of China (31600267 and 42176115) and from the Natural Science Foundation of Jiangsu Province (No. BK20201211) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, FZ., Li, XM., Meng, LH. et al. A new steroid with potent antimicrobial activities and two new polyketides from Penicillium variabile EN-394, a fungus obtained from the marine red alga Rhodomela confervoides. J Antibiot 77, 13–20 (2024). https://doi.org/10.1038/s41429-023-00666-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41429-023-00666-3