Abstract
Type 2 diabetes (T2D) is characterized by insulin resistance and reduced functional β-cell mass. Developmental differences, failure of adaptive expansion and loss of β-cells via β-cell death or de-differentiation have emerged as the possible causes of this reduced β-cell mass. We hypothesized that the proliferative response to mitogens of human β-cells from T2D donors is reduced and that this might contribute to the development and progression of T2D. Here, we demonstrate that the proliferative response of human β-cells from T2D donors in response to cdk6 and cyclin D3 is indeed dramatically impaired. We show that this is accompanied by increased nuclear abundance of the cell cycle inhibitor, p27kip1. Increasing nuclear abundance of p27kip1 by adenoviral delivery decreases the proliferative response of β-cells from non-diabetic donors, mimicking T2D β-cells. However, while both p27kip1 gene silencing and downregulation by Skp2 overexpression increased similarly the proliferative response of human β-cells, only Skp2 was capable of inducing a significant human β-cell expansion. Skp2 was also able to double the proliferative response of T2D β-cells. These studies define c-Myc as a central Skp2 target for the induction of cell cycle entry, expansion and regeneration of human T2D β-cells.
Similar content being viewed by others
Introduction
T2D (T2D) has traditionally been regarded to be the result of insulin resistance in liver, skeletal muscle and adipose tissue1,2,3. Recently, autopsy and genome-wide association studies (GWAS) suggest that it is also associated with β-cell deficiency and dysfunction4,5,6,7,8,9,10. The major factor associated with T2D is obesity, although not all obese subjects become diabetic1,3,10. In autopsy studies, patients with T2D display a reduced β-cell mass as compared to non-diabetic patients with comparable BMI. In contrast, β-cell mass is increased in non-diabetic obese subjects as compared to lean subjects8,11,12. In rodents, β-cell expansion in obesity models is associated with replication of endogenous β-cells1,3. However, there is little evidence for β-cell replication in human obesity or T2D.
In humans, understanding how the β-cell mass evolves during insulin resistance and the development of T2D is challenging due to the limitations of autopsy studies. Studies in children and young adults suggest that it is possible that some people accrue lower than average β-cell mass during their first years of development13,14. These individuals would thus require greater expansion of β-cell mass in response to insulin resistance. Indeed, β-cell mass is primarily established during the first years after birth and is highly variable among children and young adults13,14. A second possibility is that if β-cell expansion can occur in adults, some individuals may not expand their β-cell mass as effectively as others in response to obesity and insulin resistance. A third possibility is that β-cell death and/or dedifferentiation may be more prevalent in some individuals, leading to the emergence of T2D. Finally, it is likely that combinations of the above occur. In any case, the failure of β-cells to adapt to insulin resistance seems to be central to the development of T2D, whether due to reduced β-cell proliferative response, and/or increased β-cell death, and/or loss of β-cell function and de-differentiation.
A number of studies have linked the deregulation of cell cycle genes in β-cells with the development of T2D. In GWAS studies, T2D susceptibility loci have been identified in or near cell cycle genes6,7. In mouse genetic studies, the cell cycle inhibitor, p27kip1, has been linked to the development of T2D. For example, p27KIP1 progressively accumulates in the nuclei of pancreatic β-cells in T2D mouse models which lack either the insulin receptor substrate 2 (IRS2), or the leptin receptor15. In these two models of T2D, the genetic knockout of p27kip1 reduces the hyperglycemia, increases β-cell mass and maintains hyperinsulinemia, predominantly via β-cell proliferation. In addition, p27kip1 mRNA is increased in islets from human T2D donors as compared to non-diabetic donors16.
p27kip1 may be either an activator or inhibitor of cell cycle progression. In rodent β-cells, p27kip1 has been shown to be a cell cycle inhibitor17,18,19. However, in other cell types, p27kip1 has also been shown to act as an activator of cell cycle. By facilitating the formation and stabilizing the complex formed between D-cyclins and cdk4 or cdk6, p27kip1 acts as a chaperone for the assembly and nuclear translocation of the complex20. This leads to an activation of cell cycle entry. With regards to human β-cells, p27kip1 is known to be expressed in whole human islets21 and in human β-cells, mostly in their cytoplasm22,23. […]. The precise role of p27kip1 in regulating β-cell mass and proliferation is not known in humans.
p27kip1 expression is mostly regulated post-transcriptionaly by poly-ubiquitinylation and proteosomal degradation. The S-phase kinase-associated protein 2 (Skp2), a component of the SCF (Skp1-Cullin 1-F-box) E3 ubiquitin-ligase complex, has been shown to be the major p27kip1 -ubiquitin ligase. Although p27kip1 is a critical target of Skp2, many additional substrates of Skp2 has been identified. Many of these proteins, such as p21cip, p57kip2, E2F1, MEF, p130 Tob1, cyclin D1, cyclin E, Smad4 and c-myc are cell cycle regulators24. c-myc is unique among Skp2 targets, since Skp2 induces not only its ubiquitinylation, but also increases its transcriptional activity25,26,27.
While several studies have focused on β-cell loss of function and/or de-differentiation and/or susceptibility to cell death in T2D, no prior study has examined the possibility that the proliferative response of human β-cell from T2D donors is impaired. We hypothesized that this is the case. We further hypothesized that abundance of p27kip1 may be elevated in the nuclei of human T2D β-cells, limiting their response to mitogens. Finally, we hypothesized that the downregulation of p27kip1 might lead to increased proliferative response of human β-cells.
Methods
Human cadaveric islets
Human islets were provided by the NIH- and JDRF-supported Integrated Islet Distribution Program (iidp.coh.org), Prodo Lab (Irvine, CA) and the University of Alberta (Edmonton, Alberta, Canada). One hundred and six different cadaveric preparations from non-diabetic donors and six from T2D donors were used for these studies. The means age of the donors was 44.5 years ± 1.2 with a BMI of 30.9 ± 0.6 kg/M2 for the non-diabetic donors and 54.0 years ± 3 with a BMI of 29.3 ± 2.4 kg/M2 for the T2D donors. Upon arrival, islets were incubated in RPMI medium (Gibco, Grand Isle, NY) containing 5.5 mM glucose, 1% penicillin and streptomycin with 10% fetal bovine serum until they were utilized for experiments. The use of human cadaver islets was approved by and in accordance with University of Pittsburgh School of Medicine and the Icahn School of Medicine at Mount Sinai Institutional Review Boards.
Adenovirus production and transduction
Adenoviruses, all under control of the CMV promoter, were prepared using human cDNAs encoding human cdk6, human cyclins D3 and human c-Myc as described previously22,23,28,29. Ad.human Skp2 was purchased from Vector Biolabs (Philadelphia, PA). Dispersed islets on coverslips were transduced with either Ad.LacZ or relevant control adenoviruses for two hours, cultured for 72 hours as described in the Figures and immunolabeled as described below.
Islet Cell Dispersion and Immunocytochemistry
Human islets were aliquoted into 400 IEQ and washed twice in PBS and dispersed, transduced, fixed and immunolabelled as previously described18,19,24,25. Primary antisera and secondary antisera are described in Supplemental Table 1.
Immunoblotting
Islet extracts were resolved using 10% or 12% SDS-PAGE and transferred to Immobilon-P membranes (Millipore Inc., Bedford, MA). Primary antisera and secondary antisera are described in Supplemental Table 1. Each experiment shown is representative of 3–6 human islet preparations.
Assessment of β-cell mass in vitro
Human islets were dispersed as described previously18,19,24,25 and transduced with either Ad.LacZ or Ad.cdk6 + Ad.cyclinD3 or Ad.cdk6 + Ad.cyclinD3 + Ad.Skp2 or Ad.cdk6 + Ad.cyclinD3 + Ad.shp27kip1 as described previously. Ten thousand transduced islets cells from each group were plated in a high-content imaging glass bottom 96 well plate (Corning, Corning, NY), coated with poly-D lysine (Sigma-Aldrich, St Louis, MO). Five, 10, 15 and 20 thousand untransduced dispersed human islet cells were plated as controls. One day later, 50 ul of Matrigel (Corning, Corning, NY) was added to each well and cells were fixed five days after plating. Cells were then immunolabelled for insulin and DAPI as previously described18,19,24,25. The β-cell mass was assessed as previously described28.
Real-Time PCR
Human islets were dispersed as described previously and transduced with either Ad.LacZ or Ad.cdk6 + Ad.cyclinD3 or Ad.cdk6 + Ad.cyclinD3 + Ad.Skp2 or Ad.cdk6 + Ad.cyclinD3 + Ad.c-Myc as described previously. Three days after transduction, islets cells were harvested and mRNA extracted using the Absolutely RNA miniprep purification kit (Agilent Technologie, Santa Clara, CA) following the manufacturer’s instructions. cDNA and quantitative real-time PCR were performed as previously described29. Primers sequences are in Supplemental Table 2.
Statistics
Statistical analysis for Figs 1E, 2D, 3C, 4D, 5B, 6 was performed using one-way ANOVA for repeated variable with Bonferroni’s post-hoc correction. Student’s paired, two-tailed T-test was employed in Figs 1C, 3D, 4E and 7. A one-way ANOVA for repeated variable with Bonferroni’s post-hoc correction was performed for Fig 8B,C. All values are expressed as means ± SEM. “p” values < 0.05 were considered significant.
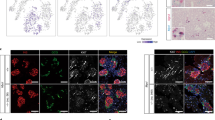
Proliferative response and p27kip1Cip/Kip expression in T2D human β-cells.
(A) Dispersed human islets from normal (Normal) or T2D (Type 2) donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and Ad.cyclin D3 (“C6 + D3”). Immunolabelling for Ki67 is shown in red, insulin in green and DAPI in blue. (B) Scattered plot of % of Ki67+/insulin + cells in response to cdk6 and cyclin D3 in non-diabetic β-cells (NL) (n = 8) and in β-cells from T2D donors (T2D) (n = 5). (C) Quantitation of % of Ki67+/insulin + cells. Bars indicate mean ± SEM. (D) Dispersed human islets from normal (Normal) (n = 7) or T2D (Type 2) (n = 6) donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and Ad.cyclin D3 (“C6 + D3”). Immunolabelling for p27kip1cip/kip is shown in red and insulin in green. (E) Quantification of data in C at 72 hours after cdk6 + cyclin D3 transduction. Bars indicate mean ± SEM of the % nuclear localization of the p27kip1 shown in insulin+ (beta) cells.
Effect of p27kip1 overexpression on human β-cell proliferation.
(A) Dispersed human islets from normal donors (n = 5) were transduced with control adenovirus (CTL) or with of Ad.p27kip1. Immunolabelling for p27kip1 is shown in red, insulin in green and DAPI in blue. (B) Quantitation of the % nuclear localization of the p27kip1 shown in insulin+ (beta) cells with increasing doses of adenovirus. Bars indicate mean ± SEM. (C) Dispersed human islets from normal donors (n = 3–5) were transduced with control adenovirus (CTL) or with cdk6 and cyclin D3 (K6 + D3), or cdk1 and cyclin A (K1 + A), or cdk1 and cyclin E (K1 + E), or cdk2 and cyclin A (K2 + A), or cdk2 and cyclin E (K2 + E), without (top panels) or with (bottom panels) Ad.p27kip1. Immunolabelling for Ki67 is shown in red, insulin in white and DAPI in blue. (D) Quantification of data in C at 72 hours after transduction. Bars indicate mean ± SEM of the % of the proliferation in each condition without Ad.p27kip1.
P27KIP1 decreases the proliferative response of human β-cells.
(A) Representative immunoblot of human islets transduced with a control adenovirus (CTL) or with Ad.cdk6 and cyclin D3 (C6 + D3), or with Ad.cdk6 and cyclin D3 with shRNA to silence p27kip1 (C6 + D3 + shp27kip1), or with shRNA to silence p27kip1 (shp27kip1). (B) Densitometric analysis of p27kip1 immunoblots. Experiments were repeated five times. *p < 0.05. (C) Dispersed human islets from normal donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and Ad.cyclin D3 (“C6 + D3”) or with Ad.cdk6 and cyclin D3 with shRNA to silence p27kip1 (C6 + D3 + shp27kip1). Immunolabelling for Ki67 is shown in red, insulin in white and DAPI in blue. (D) Quantification of data in C at 72 hours after transduction. Bars indicate mean ± SEM of the % of the proliferation with cdk6 and cyclin D3 (n = 5). (E) Dispersed human islets from normal donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and cyclin D3 with shRNA to silence p27kip1 (C6 + D3 + shp27kip1). Immunolabelling for γ-phospho-H2AX (H2AX) is shown in red, insulin in white and DAPI in blue. (F) Quantification of H2AX immunolabelling at 72 hours after transduction (n = 5). Bars indicate mean ± SEM of the % of H2AX positive β-cells.
Skp2 reduces p27kip1 expression and enhances the proliferative response of human β-cells.
(A) Dispersed human islets from normal donors were transduced with control adenovirus (“CTL”) or with Ad.Skp2. Immunolabelling for Skp2 is shown in red, insulin in green and DAPI in blue (n = 3). (B) Immunoblot of human islets transduced with a control adenovirus (Lac Z) or with Ad.cdk6 and cyclin D3 (C6 + D3), or with Ad.cdk6 and cyclin D3 and Skp2 (C6 + D3 + skp2). (C) Densitometric analysis of p27kip1 immunoblots. Experiments were repeated three to five times. *p < 0.05. (D) Dispersed human islets from normal donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and cyclin D3 (C6 + D3), or with Ad.cdk6 and cyclin D3 and Skp2 (C6 + D3 + skp2). Immunolabelling for Ki67 is shown in red, insulin in green and DAPI in blue. (E) Quantification of data in C at 72 hours after transduction. Bars indicate mean ± SEM of the % of Ki67 in insulin + cells, (n = 8). (F) Dispersed human islets from normal donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and cyclin D3 and Skp2 (C6 + D3 + Skp2). Immunolabelling for γ-phospho-H2AX (H2AX) is shown in red, insulin in white and DAPI in blue. (G) Quantification of H2AX immunolabelling at 72 hours after transduction. Bars indicate mean ± SEM of the % of H2AX positive β-cells (n = 6).
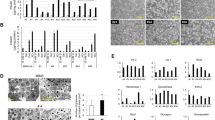
Skp2, but not p27kip1 silencing, induces the expansion of human β-cells.
(A) Representative picture-montages covering the entire wells of high imaging content 96-well plates plated with dispersed human islets cells. Dispersed human islets (ten thousand islet cells), transduced with Ad.LacZ (LacZ), Ad.cdk6 + cyclin D3 (K6 + D3), or Ad.cdk6 + cyclin D3 + Skp2 (K6 + D3 + Skp2) or Ad.cdk6 + cyclin D3 + shp27kip1 (K6 + D3 + shp27kip1) were plated and fixed five days after plating. Islet cells were immunolabelled for insulin (shown in grey). (B) Quantitation of the insulin staining area as a percentage of the control ten thousand islet cells transduced with LacZ. (n = 8).
Skp2 induces c-Myc downstream target cell cycle genes.
Dispersed human islets from normal donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and cyclin D3 (C6 + D3), or with Ad.cdk6 and cyclin D3 and Skp2 (C6 + D3 + skp2) or with Ad.cdk6 and cyclin D3 and c-Myc (C6 + D3 + c-Myc). The transduced islet cells were then harvested three days after transduction and the mRNA was extracted. Real-time qPCR was performed. Data for the D-, A, E- and B-cyclins, cdk1, 2, 4 and 6, cdc25a, E2F1, 2 and 3, p15, p21, p27kip1, p57, Skp2 and c-Myc are shown. Experiments were repeated on five to seven human islet preps.
Skp2 induces cell cycle genes in a c-Myc-dependent manner.
Dispersed human islets from normal donors were transduced with Ad.cdk6 and cyclin D3 and Skp2 (C6 + D3 + skp2, left panels) or with Ad.cdk6 and cyclin D3 and c-Myc (C6 + D3 + c-Myc, right panels) without (black bars) or with (grey bars) the c-Myc inhibitor 1RH. The transduced islet cells were then harvested three days after transduction and the mRNA was extracted. Real-time qPCR was performed. Data for the cyclin D3, E1, E2, cdk6, cdc25a, E2F1, E2F2 and E2F3 are shown. Experiments were repeated on four to five human islet preps.
Skp2 enhances the proliferative response of human β-cells in a c-Myc dependent manner and increases the proliferative response of T2D β-cells.
(A) Dispersed human islets from normal donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and cyclin D3 (C6 + D3), or with Ad.cdk6 and cyclin D3 and Skp2 (C6 + D3 + skp2) and treated with or without the c-Myc inhibitor 1RH. Immunolabeling for Ki67 is shown in red, insulin in white and DAPI in blue. Experiments were repeated on at least four human islet preps. (B) Quantification of data in C at 72 hours after transduction. Bars indicate mean ± SEM of the % of the proliferation with cdk6 and cyclin D3. (C) Dispersed human islets from T2D donors were transduced with control adenovirus (“CTL”) or with Ad.cdk6 and cyclin D3 (C6 + D3), or with Ad.cdk6 and cyclin D3 and Skp2 (C6 + D3 + skp2). Quantification of the % of Ki67 in insulin + cells at 72 hours after transduction. Bars indicate mean ± SEM of the % of Ki67 in insulin + cells. Experiments were repeated on at least three human islet preps from T2D donors. (D) Schematic cartoon of the actions of Skp2 in human β-cells. Skp2 reduces the expression of p27kip1. The reduction of p27kip1 expression alone leads to an increased proliferative capacity, but not an increased in β-cells number. Skp2 also increases, directly or indirectly (as indicated by multiple arrows and a question mark), the transcriptional activity of c-Myc, inducing the expression of cyclin E1, E2, cdc25a, E2F1 and E2F3, therefore leading to an increased proliferative capacity and expansion of human β-cells in vitro.
Results
The proliferative response of human T2D β-cells is reduced
Since β-cell mass is diminished in T2D individuals8, we explored the proliferative response of β-cells from T2D donors by overexpressing cdk6 and cyclin D3 in human dispersed islets. In this system, 14.1 ± 2.3% of normal human β-cells were labeled with Ki67 (Fig. 1). This robust response permits the proliferative response to be easily assessed. As shown in Fig. 1A–C, the proliferative response of T2D human β-cells was dramatically reduced by 80% as compared to non-diabetic β-cells.
p27kip1 nuclear abundance is increased in β-cells from T2D donors
Since p27kip1 is an important cell cycle inhibitor in rodent β-cells and since p27kip1 overexpression is linked to T2D in rodent models, we questioned whether the reduction of proliferative response in T2D human β-cells correlates with an increased level of nuclear p27kip1. As shown in Fig. 1D,E, in human β-cells from normal and T2D donors, p27KIP1 was detected more often in the nuclei of T2D β-cells as compared to normal β-cells, both under basal conditions, as well as when cell cycle entry was induced by cdk6 and cyclin D3 overexpression (Fig. 1D,E).
Introduction of p27KIP1 decreases the proliferative response of human β-cells
Next, we explored whether increased nuclear abundance of p27kip1 would decrease the proliferative capacity of human β-cells and mimic the decreased proliferative response seen in β-cells from T2D donors. Figure 2A,B shows that adenoviral expression of p27kip1 increased nuclear accumulation of p27kip1 in human β-cells to a frequency similar to those observed in β-cells from T2D donors. At 100 moi, 33.5 ± 3.7% of β-cells displayed p27kip1 in their nuclei, an abundance similar to that in β-cell from T2D donors (29.1 ± 4.5%) (Fig. 1E). In addition, p27 overexpression reduced the proliferation induced by the expression of cdk6 and cyclin D3 or any combination of G1/S cyclins and cdks (Fig. 2C,D).
p27kip1 silencing enhances the proliferative response of human β-cells
We next asked whether p27kip1 silencing would increase the proliferative response of human β-cells. As shown in Fig. 3A,B and as expected, p27kip1 expression in whole islets increased when proliferation was induced with cdk6 and cyclin D3. Silencing p27kip1 by adenoviral delivery of an shRNA targeting p27kip1 (shp27kip1) significantly decreased the expression of p27kip1, both under basal conditions and with the induction of proliferation with cdk6 and cyclin D3. Importantly, the proliferative response induced by cdk6 and cyclin D3 was significantly increased when p27kip1 was silenced (Fig. 3C,D). No significant increase in DNA damage response, measured by γP-H2AX immunolabelling, was detected (Fig. 3E,F).
Skp2 reduces p27kip1 expression and enhances the proliferative capacity of human β-cells
Since p27kip1 expression is regulated at the protein level by ubiquitinylation and proteosomal degradation30,31 and since silencing p27kip1 increased the proliferative response of human β-cells, we explored whether accelerating p27kip1 degradation would lead to increased proliferative response as well. As shown in Fig. 4A, the adenoviral delivery of Skp2 led to a marked increasing expression of Skp2 in human β-cell nuclei. In parallel, the expression of p27kip1 was increased when cdk6 and cyclin D3 were overexpressed (Fig. 4B,C). p27kip1 expression was reduced when Skp2 was overexpressed in addition to cdk6 and cyclin D3 (Fig. 4B,C). Overexpression of Skp2 alone did not lead to a significant cell cycle entry, as assessed by Ki67 immunolabelling (Fig. 4D,E). However, Skp2 expression significantly enhanced the proliferative response by cdk6 and cyclin D3 (Fig. 4D,E). The DNA damage response was not significantly increased (Fig. 4F,G).
Skp2, but not p27kip1-silencing, induces the expansion of human β-cells
While it is possible to demonstrate increases in markers of β-cell proliferation by downregulating p27kip1, it is crucial to determine whether this translates into increased numbers of human β-cells. Thus, we directly counted human β-cell number. As shown in Fig. 5A,B, the expression of Skp2 led to the appearance of greater numbers of β-cells. Surprisingly, however, silencing p27kip1 did not.
Skp2 induces c-Myc transcriptional activity
Since the overexpression of Skp2, but not the silencing of p27kip1, led to the expansion of human β-cells, we hypothesized that Skp2 might activate additional pathways besides the downregulation of p27kip1. While p27kip1 remains a major substrate of Skp2, other targets of Skp2 ubiquitinylation have been described32. Specifically, the transcriptional activity of c-Myc is stimulated by Skp2 monoubiquitinylation25,26,27. c-Myc has also been shown to be essential for the development and expansion of rodent insulinoma cells33 and modest overexpression of c-Myc, or its induction by small molecules such as harmine, are capable of inducing cell cycle entry of human β-cells33,34. Thus, we explored whether the induction of cell cycle entry and expansion by Skp2 was associated with the induction of c-Myc target genes in human β-cells. We measured the activation of cell cycle genes, known to be target genes of c-Myc transcriptional activity and controls, inducing the D-, A, E- and B-cyclins, cdks 1, 2, 4 and 6, cdc25a, E2Fs 1, 2 and 3, p15Ink4, p21cip1, p27kip1, p57kip2, Skp2 and c-Myc under control conditions (CTL), or when cdk6 and cyclin D3 were overexpressed with or without Skp2. As a positive control for c-Myc effects, we also measured the expression of the same genes when cdk6, cyclin D3 and c-Myc were overexpressed. As shown in Fig. 6, overexpression of cdk6 and cyclin D3 led to the upregulation of mRNA encoding cyclin A1 (CCNA1), cyclin E1 (CCNE1), cyclin E2 (CCNE2), E2F1 (E2F1), E2F3 (E2F3) and, as expected, cdk6 (CDK6) and cyclin D3 (CCND3). The cell cycle inhibitor, p57 (CDKN1C), was however, significantly downregulated. When Skp2 was overexpressed, cyclin E1, cyclin E2, cdc25a, E2F1 and E2F3 mRNAs were significantly further upregulated. The same mRNAs were further upregulated when c-Myc was overexpressed and their abundance was comparable to that induced by Skp2. E2F2 was the only transcript to be markedly upregulated by c-Myc but not by Skp2 cdk6 or cyclin D3.
To determine whether the upregulation of cyclin E1, cyclin E2, cdc25a, E2F1 and E2F3 by Skp2, cdk6 and cyclin D3 was dependent on c-Myc transcriptional activation, we measured their expression in presence of the c-Myc inhibitor, 1RH. As shown in Fig. 7, the Skp2-induced expression of cyclin E1, cyclin E2, cdc25a, E2F1 and E2F3 were all significantly reduced in presence of 1RH (Fig. 7, left panels). Similarly, the expression of the same genes was also significantly reduced with 1RH when c-Myc was overexpressed, with the sole exception being E2F2. Finally, as expected, the overexpression of cdk6 or cyclin D3 were not affected by the 1RH treatment.
Skp2 enhances the proliferative response of human β-cells in a c-Myc-dependent manner and increases the proliferative capacity of T2D β-cells
Since Skp2 induces the expression of cell cycle genes in a-c-Myc dependent manner, we wondered whether the proliferative effects of Skp2 were also c-Myc-dependent. As shown in Fig. 8A,B, Skp2 overexpression increased the proliferative response in human β-cells induced to cdk6 and cyclin D3. In presence of 1RH, this effect of Skp2 was markedly attenuated, but the proliferative response to cdk6 and cyclin D3 remained constant. When Skp2 was overexpressed in β-cells from T2D donors, the proliferative response almost doubled (3.7±1.4% to 8.1±1.0% for cdk6 + D3 and cdk6 + D3 + Skp2, respectively) (Fig. 8C).
Discussion
In this report, we suggest that the proliferative response of β-cells from human T2D subjects is dramatically impaired. We show that this altered proliferative response is accompanied by substantially increased nuclear abundance of the cell cycle inhibitor p27kip1 and that increasing nuclear abundance of p27kip1 decreases the proliferative response of normal β-cells from non-diabetic donors. Unexpectedly, we show that while both p27kip1 gene silencing and downregulation by Skp2 overexpression increase the proliferative response of human β-cells, only Skp2 is capable of inducing significant measurable human β-cell expansion. Skp2 was also able to double the proliferative response of T2D β-cells. We, thus, define c-Myc as a principal Skp2 target for the induction of cell cycle entry and expansion in human β-cells (Fig. 8D).
Evidence indicates that the inadequate functional β-cell mass is an essential component of T2D in humans. Autopsies from normal and T2D subjects show that the β-cell mass is reduced by about 50–65% in obese or lean T2Ds as compared to normal subjects8,9,35,36. The β-cell mass is also diminished, to a less extend, in patients with impaired fasting glucose8,35. Developmental differences, failure of adaptation and increased loss of β-cells via β-cell death or de-differentiation have emerged as the possible causes of this reduced β-cell mass. In rodents, β-cell proliferation contributes to the expansion of β-cell mass in non-diabetic obesity and a failure to proliferate and/or an increased apoptosis seem to be the features of T2D1,2,3. In humans, data suggest that the proliferation in response to obesity is more limited or absent. Studies by Butler et al. show no difference in the very low rates of β-cell proliferation among obese, lean and T2D individuals8,37. In contrast, a more recent study by Hanley et al., reports increased β-cell proliferation in obese non diabetic subjects as compared to lean non diabetics, with a significant decrease rates of replication in T2D donors38. Surprisingly, the group of Kulkarni et al. shows an increased proliferation rate in β-cells from T2Ds, as assessed by PCNA immunolabelling, together with the reduced expression of key cell cycle drivers, such as cdk239. However, no difference between the two groups was detected when the proliferation was detected using Ki67 immunolabelling in this study39. It is unclear whether this increased PCNA labeling was representative of a true replication or representative of an increased DNA damage, as reported in other cell types40.
In our study, we observe that β-cells from T2D donors have a significantly reduced proliferative response. Whether this reduced proliferative response may have contributed to developmental differences and/or failure to adapt later in life in response to an increased metabolic load, or whether this reduced proliferative response was the result of diabetes per se is not certain. Nonetheless, in the light of our findings, therapies aiming to regenerate endogenous T2D β-cells appear more challenging than anticipated.
One possible explanation for the reduced proliferative capacity in T2D human β-cells is the increased expression of p27kip1, notably in the nuclei of β-cells. Numerous studies in mice indicate that p27kip1 is a key regulator of β-cell mass. p27kip1-deficient mice display enhanced glucose tolerance, β-cell mass and proliferation19. In addition, p27kip1 accumulates in terminally differentiated non-proliferative β-cells19. Overexpression of p27kip1 during development leads to severe glucose intolerance, reduced β-cell mass and proliferation17. In mouse models of T2D, loss of p27kip1 reduces hyperglycemia by increasing β-cell mass and maintaining hyperinsulinemia, predominantly via β-cell proliferation15. Finally, inactivation of the Men1 gene acutely enhances proliferation of pancreatic islet cells, due to loss of p27kip1 and p1818. Taken together, these studies suggest that p27kip1 is a potent cell cycle inhibitor in rodent β-cells.
We have shown previously that the induction of proliferation of normal human β-cells was associated with an increased nuclear translocation of p27kip1 and that this nuclear p27kip1 was never observed in proliferating β-cells23. This suggests that p27kip1 may act as an inhibitor of cell cycle in human β-cells, but its precise role has not been explored previously. Here, we show that the level of nuclear p27kip1 is already increased under basal conditions in T2D β-cells and is dramatically further enhanced when cell cycle is activated (Fig. 1C,D). A recent study by Chen et al. shows an increased level of p27kip1 mRNA in pancreatic islets from T2D subjects41. We also show that overexpressing p27kip1 in normal β-cells to induce levels of nuclear p27kip1 similar to those detected in T2D β-cells reduces the proliferative response of human β-cells, mimicking the reduced proliferative response of T2D β-cells (Fig. 2). Altogether, these suggest that p27kip1 is a potent inhibitor of cell cycle progression in human β-cells and may contribute, at least in part, to the decreased proliferative response observed in T2D human β-cells.
Although p27kip1 silencing with shRNA or its downregulation by Skp2 both led to a similar increased proliferative response in human β-cells, only Skp2 overexpression resulted in a measurable expansion of human β-cells in vitro. This was surprising because studies described above in rodents and humans suggest that p27kip1 downregulation is important for a normal progression of β-cell cycle and that Skp2-mediated p27kip1 proteolysis is the principal regulator of p27kip1 in β-cells in mice. Like p27kip1, Skp2 is essential for β-cell development, replication and β-cell mass31. Skp2 knockout mice display accumulation of p27kip1 in β-cell nuclei. As a result, Skp2-null mice have a decreased β-cell mass, hypoinsulinemia and glucose intolerance. When challenged with a high fat regimen, Skp2 null-mice become diabetic relative to wild-type littermate, due to their inability to expand β-cell mass in response to a metabolic load31. In human islets, we show that expression of Skp2 and p27kip1 expressions are inversely correlated as expected. However, the finding that Skp2 overexpression, but not p27 silencing, induces human β-cell proliferation and expansion, suggests that Skp2 operates via an alternative and/or additional pathway beyond p27kip1 downregulation. In this regard, it was previously reported that Skp2 transcriptionally activates c-Myc via mono-ubiquitination, which then leads to the upregulation of a subset of target genes involved in the cell cycle entry and completion25,26,27. For example, in HepG2 human hepatoma cells, Skp2 induces cell cycle progression via the activation of c-Myc transcriptional activity and independently of p27kip1 expression42. In addition, c-Myc has been shown to be associated with β-cell proliferation. In rodent insulinoma cell lines, c-Myc is uniquely and specifically upregulated and is responsible in major part of their replications33. C-Myc is induced by a factor of 1.5 to 3 fold in response to glucose or partial pancreatectomy in association with rodent β-cell proliferation43,44. As another example, the induction of c-Myc by adenoviral delivery or harmine treatment drives human β-cell replication33,34. In the present study, the observation that Skp2 induces the transcription of cell cycle mRNA in a c-Myc-depend manner (Figs 6 and 7) supports that Skp2 functions as a transcriptional activator of c-Myc rather than as a component of the SCF complex responsible for p27kip1 degradation. Thus, we define c-Myc as an important target for Skp2 in expansion of human β-cells. However, whether Skp2 directly, or indirectly, activates c-Myc transcriptional activity (as shown by multiple arrows and a question mark in Fig. 8C) will require further investigation.
Previous studies have shown that high levels of c-Myc expression lead to immediate cell death and/or dedifferentiation, not only in β-cells, but also other cell types33,44,45. In our hands, however, we did not detect any DNA damage when Skp2 was overexpressed and c-Myc transcriptional activity was induced. We presume that since the induction of c-Myc activity remained moderate, it did not induce cell death, as in other studies using low levels of c-Myc overexpression or harmine in human β-cells have shown that a modest induction of c-Myc by adenoviral delivery or harmine33,34. In addition, recent studies suggest that the domain-specific ubiquitination of c-Myc by Skp2 control, not only its transcriptional activity, but also its apoptotic activity as well27. In that context, the overexpression of Skp2 is capable of inhibiting the recruitment of the tumor suppressor ARF to c-Myc transcriptional domains, leading to the inhibition of c-Myc-induced apoptosis27. Thus, one can speculate that, in human β-cells and in our hands, Skp2, not only drives c-Myc transcriptional activity and cell cycle progression, but also simultaneously inhibits c-Myc induced apoptosis via ARF inhibition. Further studies will determine whether this scenario occurs.
The findings reported here suggest that human β-cells from human T2D organ donors fail to replicate at appropriate levels and that this failure might contribute to the reduced β-cell mass in people with T2D. We show that increased nuclear abundance of p27kip1 is one possible culprit driving this reduced proliferative response. We propose that activating the Skp2-c-Myc pathway may be capable of restoring an appropriate proliferative response leading to β-cell expansion through reduction of p27 combined with direct Skp2 effects on c-Myc activation. Thus, therapies aiming at inducing the Skp2-c-Myc axis in β-cells may be beneficial for β-cells and their expansion in T2D.
Additional Information
How to cite this article: Tiwari, S. et al. Definition of a Skp2-c-Myc Pathway to Expand Human Beta-cells. Sci. Rep. 6, 28461; doi: 10.1038/srep28461 (2016).
References
Alejandro, E. U. et al. Natural history of beta-cell adaptation and failure in type 2 diabetes. Mol. Aspects Med. 42, 19–41 (2015).
Wang, P. et al. Diabetes mellitus–advances and challenges in human beta-cell proliferation. Nat. Rev. Endocrinol. 11, 201–12 (2015).
Linnemann, A. K., Baan, M. & Davis, D. B. Pancreatic beta-cell proliferation in obesity. Adv. Nutr. 5, 278–88 (2014).
Franks, P. W., Pearson, E. & Florez, J. C. Gene-environment and gene-treatment interactions in type 2 diabetes: progress, pitfalls and prospects. Diabetes Care. 36, 1413–21 (2013).
McCarthy, M. I. Genomics, type 2 diabetes and obesity. N. Engl. J. Med. 363, 2339–50 (2010).
Morris, A. P. et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44, 981–90 (2012).
Voight, B. F. et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 42, 579–89 (2010).
Butler, A. E. et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 52, 102–10 (2003).
Rahier, J. et al. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 10, Suppl 4 32–42 (2008).
Gunasekaran, U. & Gannon, M. Type 2 diabetes and the aging pancreatic beta cell. Aging. 3, 565–75 (2011).
Abdulreda, M. H., Caicedo, A. & Berggren, P. O. Transplantation into the anterior chamber of the eye for longitudinal, non-invasive in vivo imaging with single-cell resolution in real-time. JoVE. e50466 (2013).
Butler, A. E. et al. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 53, 2167–76 (2010).
Gregg, B. E. et al. Formation of a human beta-cell population within pancreatic islets is set early in life. J. Clin. Endocrinol. Metab. 97, 3197–206 (2012).
Meier, J. J. et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 57, 1584–94 (2008).
Uchida, T. et al. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat. Med. 11, 175–82 (2005).
Taneera, J. et al. Expression profiling of cell cycle genes in human pancreatic islets with and without type 2 diabetes. Mol. Cell. Endocrinol. 375, 35–42 (2013).
Rachdi, L. et al. Differential effects of p27 in regulation of beta-cell mass during development, neonatal period and adult life. Diabetes. 55, 3520–8 (2006).
Karnik, S. K. et al. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. PNAS. 102, 14659–64 (2005).
Georgia, S. & Bhushan, A. p27 Regulates the transition of beta-cells from quiescence to proliferation. Diabetes. 55, 2950–6 (2006).
Larrea, M. D. et al. Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol. Cell. Biol. 28, 6462–72 (2008).
Fiaschi-Taesch, N. et al. Survey of the human pancreatic beta-cell G1/S proteome reveals a potential therapeutic role for cdk-6 and cyclin D1 in enhancing human beta-cell replication and function in vivo. Diabetes. 58, 882–93 (2009).
Fiaschi-Taesch, N. M. et al. Human pancreatic beta-cell G1/S molecule cell cycle atlas. Diabetes. 62, 2450–9 (2013).
Fiaschi-Taesch, N. M. et al. Cytoplasmic-nuclear trafficking of G1/S cell cycle molecules and adult human beta-cell replication: a revised model of human beta-cell G1/S control. Diabetes. 62, 2460–70 (2013).
Wang, Z. et al. Skp2: a novel potential therapeutic target for prostate cancer. Biochim. Biophys. Acta. 1825, 11–7 (2012).
Kim, S. Y. et al. Skp2 regulates Myc protein stability and activity. Mol. Cell. 11, 1177–88 (2003).
von der Lehr, N. et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell. 11, 1189–200 (2003).
Zhang, Q. et al. Domain-specific c-Myc ubiquitylation controls c-Myc transcriptional and apoptotic activity. PNAS. 110, 978–83 (2013).
Tiwari, S. et al. Early and Late G1/S Cyclins and Cdks Act Complementarily to Enhance Authentic Human beta-Cell Proliferation and Expansion. Diabetes. 64, 3485–98 (2015).
Chen, H. et al. Augmented Stat5 Signaling Bypasses Multiple Impediments to Lactogen-Mediated Proliferation in Human Beta Cells. Diabetes. (2015).
Chu, I. M., Hengst, L. & Slingerland, J. M. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer. 8, 253–67 (2008).
Zhong, L. et al. Essential role of Skp2-mediated p27 degradation in growth and adaptive expansion of pancreatic beta cells. JCI. 117, 2869–76 (2007).
Kitagawa, K., Kotake, Y. & Kitagawa, M. Ubiquitin-mediated control of oncogene and tumor suppressor gene products. Cancer Sci. 100, 1374–81 (2009).
Karslioglu, E. et al. cMyc is a principal upstream driver of beta-cell proliferation in rat insulinoma cell lines and is an effective mediator of human beta-cell replication. Mol. Endocrinol. 25, 1760–72 (2011).
Wang, P. et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 21, 383–8 (2015).
Matveyenko, A. V. & Butler, P. C. Relationship between beta-cell mass and diabetes onset. Diabetes Obes. Metab. 10 (Suppl 4), 23–31 (2008).
Wang, X. et al. Regional differences in islet distribution in the human pancreas–preferential beta-cell loss in the head region in patients with type 2 diabetes. PloS One. 8, e67454 (2013).
Saisho, Y. et al. beta-cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 36, 111–7 (2013).
Hanley, S. C. et al. {beta}-Cell mass dynamics and islet cell plasticity in human type 2 diabetes. Endocrinology. 151, 1462–72 (2010).
Folli, F. et al. Altered insulin receptor signalling and beta-cell cycle dynamics in type 2 diabetes mellitus. PloS One. 6, e28050 (2011).
Mailand, N., Gibbs-Seymour, I. & Bekker-Jensen, S. Regulation of PCNA-protein interactions for genome stability. Nat. Rev. Mol. Cell Biol. 14, 269–82 (2013).
Chen, S. T., Fu, S. H., Hsu, S., Huang, Y. Y. & Hsu, B. R. Synergistic Effect of Hyperglycemia and p27(kip1) Suppression on Adult Mouse Islet Beta Cell Replication. Int. J. Endocrinol. 2012, 417390 (2012).
Li, X. et al. ERK-dependent downregulation of Skp2 reduces Myc activity with HGF, leading to inhibition of cell proliferation through a decrease in Id1 expression. Mol. Cancer Res. 11, 1437–47 (2013).
Metukuri, M. R. et al. ChREBP mediates glucose-stimulated pancreatic beta-cell proliferation. Diabetes. 61, 2004–15 (2012).
Jonas, J. C. et al. High glucose stimulates early response gene c-Myc expression in rat pancreatic beta cells. JBC. 276, 35375–81 (2001).
Laybutt, D. R. et al. Overexpression of c-Myc in beta-cells of transgenic mice causes proliferation and apoptosis, downregulation of insulin gene expression and diabetes. Diabetes. 51, 1793–804 (2002).
Acknowledgements
The authors wish to thank Christoph Buttner MD, Adolfo Garcia-Ocaña PhD, Dirk Homan MD, Donald K. Scott PhD, Sarah Stanley MD, Andrew F. Stewart MD and Rupangi C. Vasavada PhD at the Mount Sinai School of Medicine for many helpful discussions during the preparation of these studies. We also thank Dr. Rumana Huq, Deanna Benson PhD and the Microscopy Core at the Icahn school of Medicine at Mount Sinai for superb support. This work was supported by ADA Research Grant 7–12-BS-046 to NFT. Human islets were generously supplied by the NIDDK- and JDRF supported Integrated Islet Distribution Program (IIDP), by Prodo Lab (Irvine, CA) and by Dr Patrick MacDonald at the University of Alberta (Edmonton, Alberta, Canada).
Author information
Authors and Affiliations
Contributions
N.M.F.-T. and S.T. are guarantors and take full responsibility for the manuscript and its originality. N.M.F.-T. researched data, contributed to the discussion and wrote the manuscript. S.T., C.R., R.W., M.T., N.P. and P.W. researched data.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Tiwari, S., Roel, C., Tanwir, M. et al. Definition of a Skp2-c-Myc Pathway to Expand Human Beta-cells. Sci Rep 6, 28461 (2016). https://doi.org/10.1038/srep28461
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep28461
This article is cited by
-
Regenerative approaches to preserve pancreatic β-cell mass and function in diabetes pathogenesis
Endocrine (2022)
-
ZBED6 counteracts high-fat diet-induced glucose intolerance by maintaining beta cell area and reducing excess mitochondrial activation
Diabetologia (2021)
-
Neratinib protects pancreatic beta cells in diabetes
Nature Communications (2019)
-
Neuronal signals regulate obesity induced β-cell proliferation by FoxM1 dependent mechanism
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.