Abstract
Growing evidence indicates that nomogram combined with the biomarkers of systemic inflammation response could provide more accurate prediction than conventional staging systems in tumors. This study aimed to establish an effective prognostic nomogram for resectable thoracic esophageal squamouscell carcinoma (ESCC) based on the clinicopathological parameters and inflammation-based prognostic scores. We retrospectively investigated 916 ESCC patients who underwent radical esophagectomy. The predictive accuracy and discriminative ability of the nomogram were determined by concordance index (C-index) and calibration curve and compared with the 6th and 7th AJCC TNM classifications. The neutrophil lymphocyte ratio (NLR), C-reactive protein albumin (CRP/Alb) ratio, histological grade, T stage and modified N stage were integrated in the nomogram. The C-index of the nomogram for predicting the survival was 0.72, which showed better predictive ability of OS than the 6th or 7th TNM stages in the primary cohort (P < 0.001). The calibration curve showed high consistency between the nomogram and actual observation. The decision curve analysis showed more potential of clinical application of the prediction models compared with TNM staging system. Moreover, our findings were supported by the validation cohort. The proposed nomogram showed more accurate prognostic prediction for patients with ESCC after radical esophagectomy.
Similar content being viewed by others
Introduction
Esophageal cancer is a common cause of cancer death worldwide1, it is also the 5th leading cancer in incidence and 4th in mortality in China2. In 2010, there were 287,632 new cases and 208,473 deaths of esophageal cancer in China2. In despite of the development of comprehensive treatment strategies, 5-year overall survival (OS) rate for esophageal cancer is 15–35% and the prognosis remains dismal3,4. Esophageal cancer is divided into two main pathological subtypes: squamous cell carcinoma and adenocarcinoma. The predominant histological type of esophageal cancer in China is esophageal squamous cell carcinoma (ESCC), which accounts for over 90% of cases5. Traditionally, the prognosis of ESCC was performed according to the 6th and 7th edition American Joint Committee on Cancer tumor-node-metastasis (AJCC TNM) staging system6. However, ESCC patients at the same TNM stage and received similar therapy usually had variable outcomes7, suggesting that the current AJCC staging system that only assesses anatomical factors may be inadequate to make a treatment decision and evaluate the prognosis. Therefore, there is an urgent demand for a new tool that can provide reliable prognostic information in individual patient.
The visual format of nomogram is a simple and advanced prediction model that estimates the survival of individual patient by incorporating multiple clinical variables and their interdependent relationships8. The nomogram has been extensively used for many cancers and it has been proposed as an alternative or even as a new standard9,10,11,12,13,14. Recently, several studies reported that nomogram combined with the biomarkers of systemic inflammation response could provide more accurate prediction than conventional staging systems in a variety of tumors15,16,17,18. The systemic inflammation-based prognostic scores, including the Glasgow Prognostic Score (GPS), modified GPS (mGPS), C-reactive protein albumin (CRP/Alb) ratio, neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR) and lymphocyte monocyte ratio (LMR) have emerged as prognostic factors in ESCC19,20. Compared with other numerous prognostic factors, the inflammation-based prognostic scores are simple, inexpensive and widely available from preoperative evaluation of blood test. However, there is few study establishing a prognostic nomogram for ESCC based on these biomarkers. This study aimed to establish a prognostic nomogram for resectable thoracic ESCC based on the clinicopathological parameters and the inflammation-based prognostic scores, to determine whether this model provides more accurate prediction of patient survival compared with the 6th and 7th edition of AJCC TNM classifications.
Results
Clinicopathological characteristics of patients
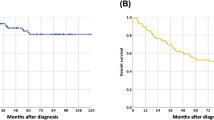
The clinicopathological characteristics of patients in the primary cohort (n = 633) and validation cohort (n = 283) are listed in Table 1. The ratio of men to women in the primary and validation cohort was 3.26:1 and 2.94:1, respectively. The median age in the primary and validation cohorts was 60 years (range, 37–83 years) and 61 years (range, 38–84 years), respectively. In the primary cohort, the median OS was 40 months (range, 3 to 146.2 months) and the rate of 3- and 5-year OS was 53.1% and 43.2%, respectively. In the validation cohort, the median OS was 44 months (range, 3 to 82 months) and the rate of 3- and 5-year OS was 54.4% and 44.6%, respectively.
Nomogram development and internal validation
In univariate analysis, histological grade, T stage, modified N stage, PLR, NLR, LMR, GPS, mGPS and CRP/Alb were found to be significant prognostic factors, while age, sex, body mass index (BMI), tumor location, tumor length and examined lymph nodes showed no statistical differences (Table 2). We explored the association among these inflammation-based prognostic scores. It was found that the classifications of GPS, mGPS and CRP/Alb were highly correlated. Thus three separate multivariate models (GPS, mGPS and CRP/Alb) were run to avoid problems with the presence of multicollinearity. Multivariate analyses demonstrated that histological grade, T stage, modified N stage, NLR, GPS, mGPS and CRP/Alb were independent risk factors for OS (Table 2). Backward stepwise selection with the Akaike information criterion (AIC) in Cox proportional hazards regression modeling was used to find a best-fit model among these independent risk factors. Finally, the nomogram that integrated five variables: histological grade, T stage, modified N stage, NLR and CRP/Alb was used to predict 3- and 5-year OS in the primary cohort (Fig. 1). The concordance index (C-index) for OS prediction was 0.72 (95% CI, 0.69–0.75). The calibration plot for the probability of survival at 3 or 5 years after surgery showed a good correlation between the prediction by nomogram and actual observation (Fig. 2A,B).
Evaluation of nomogram integrated systemic inflammation scores in the patients with esophageal squamous cell cancer after radical esophagectomy.
To use the nomogram, the value attributed to an individual patient is located on each variable axis and a line is drawn upwards to determine the number of points received for each variable value. The sum of these numbers is located on the total points axis and a line is drawn downward to the survival axis to determine the likelihood of 3- or 5-year survival. CRP/Alb, C-reactive protein/albumin; NLR: neutrophil lymphocyte ratio.
The calibration curve for predicting patient survival at 3-year (A) and 5-year (B) in the primary cohort.
Time-dependent receiver operating characteristic (ROC) curves by nomogram, 6th AJCC-TNM staging system and 7th AJCC-TNM staging system for 3-year (C) and 5-year (D) OS in the primary cohort. Decision curve analyses by nomogram, 6th AJCC-TNM staging system and 7th AJCC-TNM staging system for 3-year (E) and 5-year (F) OS in the primary cohort.
Comparison of predictive accuracy for OS between nomogram and conventional staging systems
As shown in Fig. 3, the 6th and 7th AJCC classifications showed good prognostic stratification for most patients. However, the 7th AJCC classifications were unsatisfactory in stratifying patients between stages IIIB and IIIC, while the 6th AJCC classifications were unsatisfactory in stratifying patients between stages IIA and IIB.
Our nomogram displayed better accuracy for predicting the survival in the primary cohort. The C-index of the nomogram was 0.72, which was significantly higher than that of the 7th AJCC staging system (0.68) and the 6th AJCC staging system (0.66) (P < 0.001). The time-dependent receiver operating characteristics (ROC) curve showed higher sensitivity and specificity for predicting OS at 3- and 5-year of follow-up (Fig. 2C,D). In the decision curve analysis, the nomogram demonstrated high potential of clinical application because it ensured better net benefits throughout the entire range of threshold probabilities for survival after 3 or 5 years compared with the TNM staging systems (Fig. 2E,F). These results suggest that our nomogram has better performance for predicting OS than the AJCC TNM classifications.
Validation of predictive accuracy of the nomogram for OS
Calculation of OS was done using the designed nomogram on each patient in the validation cohort. The calibration curves showed good consistency in the probability of 3- and 5-year survival between the actual observation and the nomogram prediction (Fig. 4A,B). The C-index of the nomogram for predicting OS was 0.71 (95% CI, 0.67 to 0.77) in the validation cohort, which was also significantly higher than the C-index (0.68) of the 6th TNM classification (P < 0.001) and the C-index (0.69) of the 7th TNM classification (P < 0.001). The ROC curve also showed the similar results (Fig. 4C,D). These results suggest that the nomogram is a more accurate and useful tool for the prediction of OS in patients with resectable ESCC.
The calibration curve for predicting patient survival at 3-year (A) and 5-year (B) in the validation cohort.
Time-dependent receiver operating characteristic (ROC) curves by nomogram, 6th AJCC-TNM staging system and 7th AJCC-TNM staging system for 3-year (C) and 5-year (D) OS in the validation cohort.
Discussion
In recent years, nomograms have been constructed in many malignancies and some of these nomograms have been found to be more reliable prediction than the traditional staging system9,10,11,12,13,14. Despite many advantages, there is few study on the prognostic nomogram design for resectable ESCC patients. However, that nomogram did not match well to our patient cohort. In this study, the clinicopathological variables including histological grade, T stage, modified N stage, NLR and CRP/Alb ratio were integrated in a prognostic nomogram. This nomogram predicted OS with an accuracy of C-index 0.72, which showed significantly better prediction of OS than the 6th or 7th TNM staging system in the primary cohort. The ROC curve also showed higher sensitivity and specificity for predicting 3- and 5-year OS compared with the 6th or 7th TNM staging system. These results were subsequently validated by an independent external data set. The calibration plots from both the primary and validation cohorts revealed good correlation between the predicted survival probability and the actual survival rate. The decision curve analysis showed more potential of clinical application of the prediction models compared with TNM staging system. Moreover, the information of CRP/Alb ratio and NLR can be obtained from the peripheral blood tests that were routinely conducted during preoperative examinations. Therefore our nomogram is a reliable tool to predict survival in resectable ESCC and is helpful to make individualized treatment decision.
Compared with Su’s nomogram21, our prediction model only included three clinicopathological factors: histological grade, T stage and modified N stage. Other clinicopathological factors such as tumor length and number of examined lymph nodes are not independent risk factors due to low associated hazard ratio (almost close to 1) in our study. It is worth mentioning that modified N stage instead of N stage was integrated in our nomogram. Our study found the count of examined lymph nodes was associated with the survival of the node-negative ESCC patients. Patients’ survival is positively correlated with the increasing number of negative lymph nodes for cancer examination. It is well accepted that small number of resected lymph nodes may miss positive lymph nodes and lead to the incorrect diagnosis22,23, but excessive lymphadenectomy will increase the risk of complications, such as anastomotic leakage, recurrent laryngeal nerve damage and respiratory complications24. In addition, extensive lymphadenectomy would lead to poor immune function and slow the postoperative recovery25. So the lymph node-negative ESCC patients (N0 stage patients) were divided into two groups based on examined 5 lymph nodes in our study.
Our nomogram also included the inflammation-based prognostic scores. Although the inflammation-based prognostic scores are not included in traditional staging systems, their roles in increasing predictive performance have been observed recently. The relationship between inflammation and tumor was first reported in 186326. Over the past decades, accumulating evidence has indicated that inflammation contributes to tumor growth, progression and metastasis27. Recently, the systemic inflammatory response biomarkers such as acute-phase proteins and circulating immune cells have been found to be independent markers of prognosis in a variety of cancers28,29,30,31, including ESCC19,20. Albumin and CRP are accepted markers of acute-phase proteins. The most common prognostic scores based on serum CRP and albumin concentrations are GPS and mGPS. Besides GPS and mGPS, CRP/Alb ratio can be used as an independent prognostic factor in cancer20,32. The GPS, mGPS and CRP/Alb were all independent prognostic biomarkers with high correlation in our study. However, when classified by the mGPS and GPS in our primary cohort, 86.4% and 94.3% of patients were classified in the group of score 0. In the validation cohort, more than 90% patients with a score of 0 were classified by the mGPS and GPS. Therefore, GPS and mGPS apply only to a small group of patients and have little clinical significance. Furthermore, backward stepwise selection chose the CRP/Alb ratio instead of GPS and mGPS to build the best-fit prediction model. So the CRP/Alb is superior to GPS and mGPS in our study. The PLR, NLR and LMR are the common prognostic scores based on circulating immune cells. In our study, the NLR, PLR and LMR were significant prognostic factors in univariate analysis, in while only the NLR was an independent prognostic factor for OS in multivariate analysis. Finally, the CRP/Alb ratio and NLR were included in our nomogram. Recently, Liu et al. built a nomogram based on various inflammatory biomarkers for respectable ESCC33. However, the nomogram of their study contained PLR, LMR and GPS, but not CRP/Alb radio and NLR. We found better prognostic effect of CRP/Alb radio than GPS for respectable ESCC, which was also confirmed by many recent articles20,32,34,35. For this reason, we use CRP/Alb radio instead of GPS. In addition, Liu et al. did not put the NLR included into the nomogram, because the multivariate analysis indicated the NLR not an independent prognostic factor. But significant prognostic effect was observed in the univariate analysis. This may be caused by the deficiency of correlation analysis among NLR, PLR and LMR before multivariate analysis. Moreover, Liu et al. constructed a nomogram without the necessary process of Performance and Application8,36. In this study we conducted such a supplement to make the results more scientific and reliable.
Several potential mechanisms can probably be used to explain the prognostic values of the inflammatory biomarkers in cancer: Firstly, C-reactive protein (CRP) and neutrophile granulocytes were triggered by cancer-related inflammatory factors, such as interleukin-6 (IL-6), tumor necrosis factor (TNF) and myeloid growth factors37,38. These inflammatory mediators facilitate the growth, invasion, metastasis and angiogenesis of tumor, disrupt host immune response and induce the resistance to cytotoxic drugs26,39,40. Secondly, elevated neutrophils can secrete plenty of nitric oxide, arginase and reactive oxygen species (ROS), leading to T cell activation disorders41. Meanwhile, increased circulating neutrophils have been reported to produce vascular endothelial growth factor (VEGF), causing tumor angiogenesis42,43. Thirdly, circulating monocytes determine the number of macrophages in tumor tissue and the density of tumor-associated macrophages has been proven to correlate with angiogenesis, tumor invasion and poor prognosis39,44.
Although our nomogram demonstrated good predictive accuracy for survival, there are still several limitations in this study. First, the nomogram was established based on the data from an individual institution in China. Second, our study was a retrospective study and there may exist selection bias during retrospective data collection. Third, there was heterogeneity in the reported thresholds that were used to define an elevated the inflammation-based prognostic scores in the literature. Therefore, our results need to be further verified in a prospective, large-scale collaborative study.
In conclusion, our proposed nomogram integrated the systemic inflammation scores can accurately predict the prognosis of patients with ESCC after radical esophagectomy. We believe that our nomograms would facilitate making the therapeutic decision and individualized patient counseling.
Materials and Methods
Patients
The study included 916 resectable ESCC patients who underwent radical esophagectomy in the Third Affiliated Hospital of Soochow University (Changzhou, China) from January 2002 to December 2012. Transthoracic esophagectomy with mediastinal and abdominal two-field lymphadenectomies was carried out in the present study. The inclusion criteria are as follows: radical thoracic ESCC, R0 resection, no combined malignancy, no distant metastasis, no preoperative or postoperative radiotherapy and/or chemotherapy. Among all enrolled patients, 283 patients from January 2008 to December 2009 were enrolled in the external validation cohort of this study, while the other patients were included in the primary cohort. The study protocol was performed in accordance with the guidelines outlined in the Declaration of Helsinki and was approved by the Ethics Committee of Third Affiliated Hospital of Soochow University. Written informed consent was obtained from all participants.
According to clinical findings or statistical methods, clinical data were collected and categorized as follows: age (≤60, >60), sex (male, female), BMI (<18.5, 18.5–24.5, >24.5), tumor location (upper, middle and lower), histological grade (well, moderately and poorly or not differentiated), tumor length (≤4 cm, 4–8 cm, >8 cm), T stage according to 7th edition of AJCC TNM staging (T1a-lamina propria or muscularis mucosae, T1b-submucosa, T2-superficial and deep muscular layer, T3-adventitia, T4a-pleura, pericardium, diaphragm, or adjacent, T4b-other unresectable adjacent structures), number of examined lymph nodes (≤5, 6–15, >15). To maximize the performance of the nomogram, we chose modified N stage instead of N stage. The modified N stage was defined as N stage in the 7th AJCC stage system except N0 stage. According to examined lymph nodes, the N0 stage was divided into two categories in the modified N stage. Thus the modified N stage was divided into N0 (examined lymph nodes > 5), N0 (examined lymph nodes ≤ 5), N1 (positive lymph nodes 1-2), N2 (positive lymph nodes 3–6) and N3 (positive lymph nodes ≥ 7).
All peripheral blood was collected and tested for neutrophils, lymphocytes, platelet, monocyte counts, serum C-reactive (CRP) and albumin levels just before operation. The inflammation-based prognostic scores in this study were defined and calculated as follows: (1) GPS, patients with both CRP >10 mg/L and albumin <35 g/L were allocated a score of 2; patients with CRP > 10 mg/L or albumin <35 g/L were allocated a score of 1; and patients with both CRP <10 mg/L and albumin>35 g/L were allocated a score of 0. (2) mGPS, patients with CRP < 10 mg/L were allocated a score of 0; patients with CRP > 10 mg/L or albumin >35 g/L were allocated a score of 1; patients with both CRP > 10 mg/Land albumin< 35 g/L were allocated a score of 2. Optimal cutoff values including NLR (NLR≤1.7, NLR>1.7), PLR (PLR ≤ 120, PLR > 120), LMR (LMR ≤ 3.57, LMR > 3.57) and CRP/Alb (CRP/Alb ≤ 0.06, 0.06 < CRP/Alb ≤ 0.12, CRP/Alb > 0.12) were determined by using X-tile software (http://www.tissuearray.org/rimmlab)45.
Follow-Up
Follow-up was conducted as previously described46. All patients were followed up every three months in the first 2 years, every six months until 5 years and then once annually. All patients underwent clinical, laboratory, imaging and endoscopy examinations for assessing recurrence or metastasis.
The latest follow-up was conducted at the end of December 2014. All patients were followed up by phone calls and regular letters. The observation time in this study was the interval from the date of surgical resection to death or latest follow-up. Survived patients were censored on the day of the last follow-up. OS was determined by the period from the time of surgery to the last follow- up or date of patient death. The median follow-up was 39 months (range, 3 to 146.2 months).
Statistical Analysis
Statistical analysis was carried out using SPSS 17.0 for windows (SPSS, Chicago, IL) and R software version 3.2.0 (http://www.r-project.org/) with Hmisc, rms and survival ROC packages. Survival curves were made using the Kaplan-Meier method and compared using the log-rank test. All variables that achieved significance at P < 0.05 in univariate analyses were enrolled in multivariate Cox’s proportional hazards model. The nomogram was formulated based on the results of multivariate analysis. A final model selection was performed using a backward stepdown selection process with the AIC47. To evaluate the nomogram performance, we assessed both the discrimination and calibration of these models. The analysis of time-dependent ROC curve and C-index were used to compare the discrimination power for OS between different models. Confidence intervals (CIs) were obtained by creating 1000 bootstrap samples from the entire dataset and replicating the estimation process. The larger the C-index, the more accurate was the prognostic prediction48. Decision curve analysis was used to evaluate the clinical application of prediction models by quantifying the net benefits49. During the external validation of the nomogram, the total points of each patient in the validation cohort were calculated according to the generated nomogram, then Cox regression in this cohort was performed using the total points as a factor and the C-index and calibration curve were finally derived based on the regression analysis. Nomogram construction and validation were performed with nomogram guide8,36. A P value less than 0.05 was considered to be statistically significant unless otherwise specified.
Additional Information
How to cite this article: Shao, Y. et al. Prognostic nomogram integrated systemic inflammation score for patients with esophageal squamouscell carcinoma undergoing radical esophagectomy. Sci. Rep. 5, 18811; doi: 10.1038/srep18811 (2015).
References
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015).
Chen, W., Zheng, R., Zeng, H., Zhang, S. & He, J. Annual report on status of cancer in China, 2011. Chin J Cancer Res 27, 2–12 (2015).
Enzinger, P. C. & Mayer, R. J. Esophageal cancer. N Engl J Med 349, 2241–2252 (2003).
Pennathur, A., Gibson, M. K., Jobe, B. A. & Luketich, J. D. Oesophageal carcinoma. Lancet 381, 400–412 (2013).
Law, S. & Wong, J. Changing disease burden and management issues for esophageal cancer in the Asia-Pacific region. J Gastroenterol Hepatol 17, 374–381 (2002).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17, 1471–1474 (2010).
Reeh, M. et al. An attempt at validation of the Seventh edition of the classification by the International Union Against Cancer for esophageal carcinoma. Ann Thorac Surg 93, 890–896 (2012).
Balachandran, V. P., Gonen, M., Smith, J. J. & DeMatteo, R. P. Nomograms in oncology: more than meets the eye. Lancet Oncol 16, e173–180 (2015).
Graesslin, O. et al. Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 28, 2032–2037 (2010).
Han, D. S. et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 30, 3834–3840 (2012).
Wang, Y. et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 31, 1188–1195 (2013).
Yang, L., Shen, W. & Sakamoto, N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol 31, 468–474 (2013).
Sternberg, C. N. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol 24, 3819–3820 (2006).
Touijer, K. & Scardino, P. T. Nomograms for staging, prognosis and predicting treatment outcomes. Cancer 115, 3107–3111 (2009).
Deng, Q. et al. Prognostic value of pre-operative inflammatory response biomarkers in gastric cancer patients and the construction of a predictive model. J Transl Med 13, 66 (2015).
Ku, J. H. et al. The prognostic value of pretreatment of systemic inflammatory responses in patients with urothelial carcinoma undergoing radical cystectomy. Br J Cancer 112, 461–467 (2015).
Szkandera, J. et al. The lymphocyte/monocyte ratio predicts poor clinical outcome and improves the predictive accuracy in patients with soft tissue sarcomas. Int J Cancer 135, 362–370 (2014).
Szkandera, J. et al. Validation of the prognostic relevance of plasma C-reactive protein levels in soft-tissue sarcoma patients. Br J Cancer 109, 2316–2322 (2013).
Dutta, S., Crumley, A. B., Fullarton, G. M., Horgan, P. G. & McMillan, D. C. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg 35, 1861–1866 (2011).
Wei, X. L. et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 15, 350 (2015).
Su, D. et al. Prognostic Nomogram for Thoracic Esophageal Squamous Cell Carcinoma after Radical Esophagectomy. PLoS One 10, e0124437 (2015).
Hulscher, J. B. et al. Prospective analysis of the diagnostic yield of extended en bloc resection for adenocarcinoma of the oesophagus or gastric cardia. Br J Surg 88, 715–719 (2001).
Nigro, J. J. et al. Node status in transmural esophageal adenocarcinoma and outcome after en bloc esophagectomy. J Thorac Cardiovasc Surg 117, 960–968 (1999).
Jamieson, G. G., Lamb, P. J. & Thompson, S. K. The role of lymphadenectomy in esophageal cancer. Ann Surg 250, 206–209 (2009).
Paik, K. Y., Lee, I. K., Lee, Y. S., Sung, N. Y. & Kwon, T. S. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat 46, 65–73 (2014).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Proctor, M. J. et al. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer 107, 695–699 (2012).
Sun, K., Chen, S., Xu, J., Li, G. & He, Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 140, 1537–1549 (2014).
Paramanathan, A., Saxena, A. & Morris, D. L. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol 23, 31–39 (2014).
McMillan, D. C. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39, 534–540 (2013).
Kinoshita, A. et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 22, 803–810 (2015).
Liu, J. S., Huang, Y., Yang, X. & Feng, J. F. A nomogram to predict prognostic values of various inflammatory biomarkers in patients with esophageal squamous cell carcinoma. Am J Cancer Res 5, 2180–2189 (2015).
Xu, X. L., Yu, H. Q., Hu, W., Song, Q. & Mao, W. M. A Novel Inflammation-Based Prognostic Score, the C-Reactive Protein/Albumin Ratio Predicts the Prognosis of Patients with Operable Esophageal Squamous Cell Carcinoma. PLoS One 10, e0138657 (2015).
Zhou, T. et al. Ratio of C-Reactive Protein/Albumin is An Inflammatory Prognostic Score for Predicting Overall Survival of Patients with Small-cell Lung Cancer. Sci Rep 5, 10481 (2015).
Iasonos, A., Schrag, D., Raj, G. V. & Panageas, K. S. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26, 1364–1370 (2008).
Morris-Stiff, G., Gomez, D. & Prasad, K. R. C-reactive protein in liver cancer surgery. Eur J Surg Oncol 34, 727–729 (2008).
Kuper, H., Adami, H. O. & Trichopoulos, D. Infections as a major preventable cause of human cancer. J Intern Med 248, 171–183 (2000).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Diakos, C. I., Charles, K. A., McMillan, D. C. & Clarke, S. J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15, e493–503 (2014).
Muller, I., Munder, M., Kropf, P. & Hansch, G. M. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol 30, 522–530 (2009).
Kusumanto, Y. H., Dam, W. A., Hospers, G. A., Meijer, C. & Mulder, N. H. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis 6, 283–287 (2003).
Shamamian, P. et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol 189, 197–206 (2001).
Mantovani, A., Schioppa, T., Porta, C., Allavena, P. & Sica, A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 25, 315–322 (2006).
Camp, R. L., Dolled-Filhart, M. & Rimm, D. L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10, 7252–7259 (2004).
Ning, Z. H. et al. Proposed Modification of Nodal Staging as an Alternative to the Seventh Edition of the American Joint Committee on Cancer Tumor-Node-Metastasis Staging System Improves the Prognostic Prediction in the Resected Esophageal Squamous-Cell Carcinoma. J Thorac Oncol 10, 1091–1098 (2015).
Harrell, F. E., Jr., Lee, K. L. & Mark, D. B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy and measuring and reducing errors. Stat Med 15, 361–387 (1996).
Huitzil-Melendez, F. D. et al. Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 28, 2889–2895 (2010).
Vickers, A. J. & Elkin, E. B. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 26, 565–574 (2006).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81171653 and 81301960).
Author information
Authors and Affiliations
Contributions
Y.J.S., Z.H.N., H.L.P. and J.T.J. conceived and designed the study and helped to draft the manuscript. Y.P.S. performed the statistical analysis. J.C., Y.T.G., W.D.G. and J.H. performed the data collection. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Shao, Y., Ning, Z., Chen, J. et al. Prognostic nomogram integrated systemic inflammation score for patients with esophageal squamouscell carcinoma undergoing radical esophagectomy. Sci Rep 5, 18811 (2015). https://doi.org/10.1038/srep18811
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep18811
This article is cited by
-
Prognostic value of the combined preoperative plasma fibrinogen and systemic inflammatory indexes in ESCC patients
Discover Oncology (2023)
-
The Clinical Impacts of Neutrophil to Lymphocyte Ratio for Esophageal Cancer Patients Who Receive Curative Treatment
Indian Journal of Surgery (2022)
-
The value of complete blood count for the prognosis analysis of preoperative esophageal squamous cell carcinoma
BMC Cancer (2021)
-
Prognostic value of preoperative peripheral blood mean platelet volume/platelet count ratio (MPV/PC) in patients with resectable cervical cancer
BMC Cancer (2021)
-
Prognostic role of pre-treatment C-reactive protein/albumin ratio in esophageal cancer: a meta-analysis
BMC Cancer (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.