Abstract
Clinical studies aimed at identifying effective and simple prognostic markers for gastric cancer are still being carried out. Inflammatory prognostic index (IPI) is being recognized as a promising prognostic marker in patients with Non-Small Cell Lung Cancer. To evaluate the prognostic utility of IPI in stage 4 gastric cancer. A total of 152 patients with stage 4 gastric cancer, whose laboratory parameters, progression-free survival (PFS) and overall survival (OS) data could be accessed, were evaluated. Kaplan Meier analysis was used for survival analyses. Hazard ratios were expressed with 95% CI values. All methods were performed in accordance with the relevant guidelines and regulations. Study was approved by the Manisa Celal Bayar University’s Non-Invasive Clinical Research Ethics Committee (approval No. E-85252386-050.04.04-49119, date: 22.03.2021). We confirm that all methods were performed in accordance with relevant named guidelines and regulations. Median age at diagnosis was 63 years (range: 32–88). The number of patients who received first-line chemotherapy was 129 (84.9%). Median PFS with first-line treatment was 5.3 months, while it was 3.3 months with second-line treatment. Median OS was 9.4 months. Median IPI score was 22.2. We evaluated IPI score for its value in detecting survival status with ROC analysis and identified an IPI cut-off score of 14.6. Low IPI score was significantly associated with longer PFS and OS compared to high IPI (PFS in high vs. low IPI, 3.6 vs. 7 months; p < 0.001) (OS in high vs. low IPI, 6.6 vs. 14.2 months; p < 0.001). IPI score can be an independent prognostic index that is inexpensive, easy to access and evaluate for patients with metastatic gastric cancer, and may be useful in predicting survival in daily practice.
Similar content being viewed by others
Introduction
Gastric cancer is the 5th most common cancer and the 3rd most common cause of cancer-related death1,2. Cancer-associated systemic inflammatory response is triggered by tumor microenvironment and is associated with tumor development, invasion, and metastasis3. Systemic inflammatory response is associated with predisposition to cachexia and deterioration of the patient’s performance4. Some inflammatory biomarkers generated using hematological and biochemical parameters have value in predicting adverse effects such as decreased overall or progression-free survival (OS, PFS) or resistance to chemotherapy, and thus, inflammatory biomarkers have been investigated in a number of tumor types5,6. The link between inflammation and cancer is very strong and just as complex. The interaction of cells responsible for inflammation and tumor cells varies according to suppressors or activators at various steps. Inflammation plays an important function in tumor development, invasion and metastasis. C-reactive protein (CRP), which is produced by liver cells, is an acute phase protein regulated by IL-1, IL 6 and tumor necrosis factor7. Many studies have shown that increased CRP is negatively associated with prognosis. Also, neutrophil/lymphocyte ratio (NLR) has been associated with poor prognosis in various cancers, and some studies have confirmed that NLR is a reliable marker in this respect.
Significant variation in these associations has been observed, and the sources of this variation are poorly understood. NLR may be demonstrative of the balance between the harmful effects of neutrophils and the beneficial effects of adaptive immunity8. Nonetheless, the prognostic potential of NLR may not be the same in all patient subgroups and in all solid tumors9.
Clinical studies aimed at determining effective and simple prognostic markers for gastric cancer are still being carried out. Various combinations of parameters, such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) or the Glasgow prognostic score, have been used10,11. Subsequently, increasing evidence shows that prognostic markers can be used to discern prognosis in various malignant tumors. Inflammatory prognostic index (IPI), a measure based on CRP, NLR and serum albumin, has emerged as a promising prognostic marker in patients with Non-Small Cell Lung Cancer12.
In our study, we aimed to investigate whether IPI values are associated with PFS and OS in patients with stage-4 gastric cancer.
Methodology
Patients and methods
In our study, patients who were diagnosed with metastatic gastric cancer between 2013 and 2020 at Manisa Celal Bayar University Hospital and Private İzmir Kent Hospital were retrospectively analyzed. A total of 152 patients with stage 4 gastric cancer whose laboratory parameters, PFS data and OS data could be accessed, were evaluated. The blood tests of the patients were performed in the laboratory of the institute where they were treated. Pre-treatment parameters were addressed so that the administered treatments did not affect laboratory and performance results. The first blood tests were obtained before the treatment. Laboratory parameters, performance status, age at diagnosis, sex and treatments of the patients who applied to the oncology outpatient clinic in both institutes were recorded. Since it is easier to implement, the performance status of the patients was evaluated with ECOG performance scoring.
The present study was approved by the Manisa Celal Bayar University Faculty of Medicine ethics committee/institutional review board (approval No. E-85252386-050.04.04-49119, date: 22.03.2021) and the need of informed consent was waived by Manisa Celal Bayar University Faculty of Medicine ethics committee due to the retrospective study. We confirm that all methods were performed in accordance with relevant named guidelines and regulations.
During the evaluation of laboratory tests, CEA and CA19-9 were quantified from the peripheral blood samples of patients using the Cobas 6000 e 601 module (Roche Diagnostics, Switzerland) and Beckman Unicel DXL 800 (Beckman Coulter Diagnostics, United States) devices. Serum albumin, creatinine, lactate dehydrogenase (LDH), serum calcium and CRP levels were investigated using the Cobas 6000 c 501 Chemistry System (Roche Diagnostics, Switzerland) and Beckman AU 5800 Chemistry System (Beckman Coulter Diagnostics, United States). Hemogram tests (hemoglobin, platelet, neutrophil, lymphocyte) were performed using the Sysmex Xn-1000 (Sysmex Diagnostics, Japan) and Mindray BC 6800 (Mindray medical, China) devices. In both institutes, the units of the tests and reference ranges of the examined parameters were the same. The NLR and PLR values were calculated using the individual counts from peripheral blood. As in the original study, IPI was calculated using the formula: CRP × NLR/serum albumin.
OS was calculated from the date of metastatic disease diagnosis to the date of death or the date of last follow-up. PFS was calculated as the interval between the date of diagnosis and progression or death. Disease progression was accepted as imaging compatible with progressive disease on CT or PET-CT according to the response evaluation criteria in solid tumors version 1.1 (RECIST v1.1).
Statistical analysis
Categorical variables are presented in tables as patient numbers and percentages. Comparisons were performed with odds ratios (OR) and corresponding 95% confidence intervals (CI) using chi-square or Fisher's exact test. Kaplan Meier analysis was used to depict survival curves. Univariate and multivariate analyzes were performed using the Cox proportional hazards model to assess the survival difference. Chi-square tests were used to analyze the relationship between NLR, PLR, IPI and clinicopathological parameters. Only variables that were significant as prognostic parameters in the univariate Cox's proportional hazards model were included in the multivariable analysis to identify independent prognostic factors. Variables which were added in the multivariate cox regression analysis were assessed with spearman rank correlation test to exclude multicollinearity. None of the bivariate correlation coefficients were above 0.7, which in turn indicated lack of significant multicollinearity. ROC analysis was used to determine the discriminative power of IPI score and to identify discriminatory cut-off. All statistical evaluations were a p value of 0.05 was considered statistically significant. All data were analysed using a commercially available software program (IBM SPSS statistics for windows, version 22.0, Armonk, NY: IBM corp).
Ethical approval
Study was approved by the Manisa Celal Bayar University’s Non-Invasive Clinical Research Ethics Committee (approval No. E-85252386-050.04.04-49119, date: 22.03.2021).
Patient consent
Since the study was a retrospective archive search, informed consent was not obtained from the patients.
Results
A total of 152 patients were included for analysis in the present study. All patients had stage 4 disease. Median age at diagnosis was 63 years (range: 32–88). 30.9% (n = 47) of the patients were female, 69.1% (n = 105) were male. Demographic data are given in Table 1 and clinical features are given in Table 2.
It was found that 83.6% (n = 127) of the patients had de novo metastatic disease. Twenty-three (15.1%) patients had an ECOG performance score of 3–4, and none of these patients received chemotherapy. The number of patients who received chemotherapy as first-line treatment was 129 (84.9%) and 67 (44.1%) patients received chemotherapy as second-line therapy. Overall, 143 (94.1%) of the patients had died at the time of data collection.
There were 45 (29.6%) patients who had previously undergone palliative or curative surgery for gastric cancer, while 107 (70.4%) were not operated. The number of Cerbb2 positive cases was 14 (9.2%). All of these cases received trastuzumab together with chemotherapy during first-line treatment.
Median PFS1 was 5.3 months, PFS2 was 3.3 months, and OS was 9.4 months. OS was 2.2 months in patients with ECOG 3–4, while it was 11 months in patients with ECOG 0–1-2 (p < 0.001). (mOS) is 11.9 months in patients with 2 or less metastatic areas, and 7.5 months in patients with 3 or more (p = 0.028).
The IPI value ranged from 0.2 to 38.7 (median, 22.2), and NLR ranged from 0.41 to 36.00 (median, 3.61).
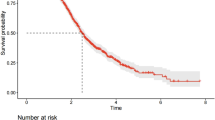
We evaluated IPI for its predictive value in survival status with ROC analysis and identified the cut-off value as 14.6. Low IPI score was significantly associated with longer OS and PFS compared to high IPI score (median OS in high vs. low IPI, 6.6 vs. 14.2 months; p < 0.001) (median PFS in high vs. low IPI, 3.6 vs. 7 months; p < 0.001). Survival function patterns by IPI low and IPI high groups are shown in Fig. 1. We evaluated NLR for its predictive value in survival status and identified the cut-off value as 3. Low NLR score was significantly associated with longer OS compared to high NLR score (median OS in high vs. low IPI, 7.2 vs. 13.6 months; p < 0.001) (median PFS in high vs. low NLR, 3,5 vs. 6.6 months; p < 0.001). Survival function patterns by NLR low and NLR high group has shown in Fig. 2. Notably, IPI classification were identified as an independent prognostic factor for OS and PFS.
The number of patients who received chemotherapy as a monotherapy (capecitabine, 5-FU, taxane) or doublet regimen (FOLFOX, XELOX, Platin-5-FU, FOLFIRI, Platin-Taxane, Taxane-5FU) was 59.7% (77), triplet regimen (FLOT, DCF, ECF) recipients represented 40.3% (52). While 42.9% (33) of 77 patients who received monotherapy or doublet regimen as first-line chemotherapy were in the IPI low group, 57.1% (44) were in the IPI high group. While 48.1% (25) of 52 patients who received triplet chemotherapy regimen were in the IPI low group, 51.9% (27) were in the IPI high group. There was no significant difference between the groups (p = 0.56).
While 48.1% (37) of 77 patients who received monotherapy or doublet regimen as first-line chemotherapy were in the NLR low group, 51.9% (40) were in the NLR high group. While 42.3% (22) of 52 patients who received triplet chemotherapy regimen were in the NLR low group, 57.7% (30) were in the NLR high group. There was no significant difference between the groups (p = 0.52).
While 53.2% (41) of 77 patients who received monotherapy or doublet regimen as first-line chemotherapy were in the PLR low group, 46.8% (36) were in the PLR high group. While 46.2% (24) of 52 patients who received triplet chemotherapy regimen were in the PLR low group, 53.8% (28) were in the PLR high group. There was no significant difference between the groups (p = 0.43).
Univariate and multivariable analyses of OS and PFS by prognostic factors are summarized in Tables 3 and 4.
Discussion
Three inflammatory markers are very important for prediction of survival in gastric cancer. These are NLR, CRP and serum albumin level13. There are several prognostic marker systems that allow the interpretation of prognosis based on inflammation in solid malignant tumors. As mentioned, high NLR has been identified as an unfavorable prognostic factor in various cancers. IL-1, IL-6 and tumor necrosis factor-α are at the root of the increase in CRP, an acute phase inflammation protein. CRP is also known to contribute to aggressive cancer behavior, and it has been established as a factor of poor prognosis in various solid tumor types14.
Hypoalbuminemia may be another important variable as it has been shown to be significantly associated with shorter survival time in cancer patients. Hypoalbuminemia and high CRP levels are frequently observed in advanced cancer patients and are generally reported to be associated with worse survival15. This may be associated with the fact that protein digestion and absorption are decreased in patients with gastric cancer, resulting in a negative nitrogen balance16. For this reason, it is thought that the IPI, which is the combination of these three important parameters, can serve as a more effective scoring system in predicting the prognosis of cancer patients. A higher IPI score indicates more severe inflammation and a weaker immune response in patients.
In this analysis of 152 gastric cancer patients, we found that NLR and IPI were associated with survival status, as measured by OS and PFS. The IPI is a reproducible, cost-effective and available prognostic marker for gastric cancer patients17. Several prognostic scores have been developed to aid in the assessment of cancer prognosis, some of which are based on serum CRP and albumin levels, such as the Glasgow prognostic score. Similar to IPI, Glasgow prognostic score scores demonstrating high inflammation and poor immune response have been shown to be associated with worse prognosis in a variety of different tumor types12,18. We propose that patients with a high NLR and high IPI score should thus be recognized as a high-risk group in terms of progression and survival.
An important previously published study demonstrated that CRP/albumin ratio and NLR serve as independent prognostic factors for OS in patients with gastric cancer19. In the present study, Cox regression analysis demonstrated that the IPI score could be used as a predictor for both OS and PFS. There are studies showing that inflammatory indices such as NLR and systemic immune inflammation index can predict treatment response and are associated with chemotherapy resistance20,21. IPI similarly appears to be able to predict both treatment response and OS since IPI was associated with PFS and OS regardless of the preferred chemotherapy regimen. Also, IPI seems to be a more sensitive index compared to NLR in the prediction of PFS and OS.
Since this study was a retrospective two-center study with a relatively small number of patients, it would be appropriate to confirm these results with prospective studies with larger patient participation.
Conclusion
As a result of cox regression analysis, it was determined that IPI score could be a parameter that could predict prognosis and survival in patients with metastatic gastric cancer. The IPI score can be an independent prognostic index that is inexpensive, easy to access and evaluate for metastatic gastric cancer patients, and may be useful in predicting survival in daily clinical practice.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Crew, K. D. & Neugut, A. I. Epidemiology of gastric cancer. World J Gastroenterol: WJG 12(3), 354 (2006).
Hines, R. B. et al. Post-treatment surveillance testing of patients with colorectal cancer and the association with survival: Protocol for a retrospective cohort study of the Surveillance, Epidemiology, and End Results (SEER)-Medicare database. BMJ Open 8(4), e022393 (2018).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140(6), 883–899 (2010).
Allgayer, H., Heiss, M. & Schildberg, F. Prognostic factors in gastric cancer. J. Br. Surg. 84(12), 1651–1664 (1997).
Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340(6), 448–454 (1999).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454(7203), 436–444 (2008).
Mocellin, M. C. et al. Fish oil decreases C-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids 48(9), 879–888 (2013).
Zahorec, R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 102(1), 5–14 (2001).
Templeton, A. J. et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. JNCI J. Natl. Cancer Inst. 106(6), dju124 (2014).
Jin, J., Hu, K., Zhou, Y. & Li, W. Clinical utility of the modified Glasgow prognostic score in lung cancer: A meta-analysis. PLoS ONE 12(9), e0184412 (2017).
Okada, S. et al. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann. Thorac. Surg. 104(1), 296–302 (2017).
Dirican, N. et al. A new inflammatory prognostic index, based on C-reactive protein, the neutrophil to lymphocyte ratio and serum albumin is useful for predicting prognosis in non-small cell lung cancer cases. Asian Pac. J. Cancer Prevent.: APJCP 17(12), 5101 (2016).
Formica, V. et al. Gastric inflammatory prognostic index (GIPI) in patients with metastatic gastro-esophageal Junction/Gastric Cancer treated with PD-1/PD-L1 Immune checkpoint inhibitors. Target. Oncol. 15, 327–336 (2020).
Yu, Q. et al. Prognostic role of C-reactive protein in gastric cancer: A meta-analysis. Asian Pac. J. Cancer Prev. 14(10), 5735–5740 (2013).
Crumley, A. B., Stuart, R. C., McKernan, M. & McMillan, D. C. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer?. World J. Surg. 34(10), 2393–2398 (2010).
Heneghan, H. M. et al. Prospective study of malabsorption and malnutrition after esophageal and gastric cancer surgery. Ann. Surg. 262(5), 803–808 (2015).
Yamanaka, T. et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 73(3–4), 215–220 (2007).
Ishizuka, M., Nagata, H., Takagi, K. & Kubota, K. Influence of inflammation-based prognostic score on mortality of patients undergoing chemotherapy for far advanced or recurrent unresectable colorectal cancer. Ann. Surg. 250(2), 268–272 (2009).
Mao, M. et al. C-reactive protein/albumin and neutrophil/lymphocyte ratios and their combination predict overall survival in patients with gastric cancer. Oncol. Lett. 14(6), 7417–7424 (2017).
Yilmaz, M., Baran, A. & Yilmaz, M. K. Predictive significance of inflammatory indexes in metastatic nonsmall cell lung cancer patients treated with platinum-doublet chemotherapy. J. Cancer Res. Ther. 18(1), 220 (2022).
Sun, H., Hu, P., Du, J. & Wang, X. Predictive value of inflammatory indexes on the chemotherapeutic response in patients with unresectable lung cancer: A retrospective study. Oncol. Lett. 15(3), 4017–4025 (2018).
Author information
Authors and Affiliations
Contributions
A.O, A.P.E. and F.E. collected the patients data, A.O. had done the statistical analysis and wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ozveren, A., Erdogan, A.P. & Ekinci, F. The inflammatory prognostic index as a potential predictor of prognosis in metastatic gastric cancer. Sci Rep 13, 7755 (2023). https://doi.org/10.1038/s41598-023-34778-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-34778-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.