Abstract
Prostate cancer is the second most common cancer with sexual history as a consistent risk factor. This is the pioneering study that evaluates the frequency of HPV infection in prostate cancer in India. Ninety five (95) histopathologically confirmed cancer and fifty five (55) BPH from Indian population were analyzed for HPV infection using a pair of consensus sequence primer followed by type specific PCRs for both high-risk and low-risk HPV types. The data demonstrate HPV infection in 41% of prostate tumor biopsies and 20% in BPH. Subsequent PCR- based HPV typing using type - specific primers revealed 32% were infected with HPV type 16 whereas 6% were found to be positive for HPV type 18, while in BPH controls only 5% of the BPH controls were infected with HPV 16 and this difference was highly significant (p = 0.0004). Significant proportion of HPV infected (74%) cases belonged to stage III and IV (p < 0.001) with a high Gleason score ≥8 (p = 0.003). The study represents for the first time the incidence of HPV infection in prostate cancer in Indian population and strengthens the hypothesis that HPV infection could be one of the co factor associated with progression of prostate cancer.
Similar content being viewed by others
Introduction
Prostate cancer is a common urological malignancy and an important health concern among males is India1,2,3. The exact mechanisms of the progression of prostate gland into a cancer are not well characterized. The growing epidemiological studies have suggested that prostate tissue is prone to sexually transmitted infection with several viruses having oncogenic potential such as polyomaviruses (SV40), human papillomaviruses (HPVs) and members of the herpes virus family4,5,6,7,8,9. It has also been implicated that cellular transformation in prostate cancer is carried out by viral oncogenes of polyomaviruses, or the E6 and E7 proteins of HPVs10. Human papillomavirus is a small, non-enveloped DNA virus with a circular, double stranded DNA genome of approximately 8 Kb genome size. The oncogenic potential of HPV is determined by E6 and E7 oncogenes that interact with and inhibit the activities of critical components of cell-cycle regulatory systems, in particular E6 with p53 and E7 with Rb11,12.
In our previous studies we have reported that oncogenic subtypes of HPV, with the most common types 16 and 18, have a strong association with cervical cancer13,20,21. It has been speculated that cervical and prostate cancer may represent, in some aspects, homologous cancers in females and males, respectively. Both of the cancer types are influenced by similar factors like sexual activities and infection status14 and are most commonly occurring cancers in the developing countries like India, with a roughly equal lifetime risk. There is growing evidence of the role of the sexually transmitted diseases in prostate cancer9. Therefore, it is possible that there would be an association between oncogenic HPV infection and prostate cancer. Studies have reported the contrasting role of HPV infection in the pathogenesis of prostate cancer15,16,17,18. Molecular investigations have reported that E6/E7 oncoprotein of HPV types 16 or 18 can immortalize prostate epithelial cells17,18. The meta-analysis studies have demonstrated that HPV infection was associated with increased prostate cancer risk4,18. On the contrary certain number of studies has also reported that HPV infection is absent in prostate cancer16,37,38.
The inconsistent results across many of these epidemiologic studies could possibly be related to the variation in ethnicity and population of one geographic region to another. Based upon the above findings, the current pioneering study is designed to analyze the HPV infection status in prostate cancer cases in comparison with benign prostate hyperplasia (BPH) controls and to find its correlation if any with the various risk factors like mean age, PSA level, gleason score and disease aggressiveness19 in the North Indian population.
Methods
In the present study, a total of one hundred and fifty (n = 150) histopathologically confirmed, prostate biopsies comprising 95 cancer cases and 55 Benign prostate hyperplasia (BPH) were collected from the Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh. Only clinically confirmed cases comprising 76% patients of stages I & II and 24% of stage III & stage IV were processed for DNA extractions. Most of the prostate cancer cases belonged to the age group of 60–79 years with average age of 68 years (Table 1).
Written informed consent was obtained from all the subjects included in the study and was carried out in accordance with the principles of the Helsinki Declaration. The study was approved by the Ethics Committee of the Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh (India).
Extraction and quantitation of genomic DNA
High molecular-weight genomic DNA was isolated from tumor tissue and BPH samples that served as controls by standard Proteinase-K digestion and phenol-chloroform extraction procedure routinely followed in our lab20. The concentration of genomic DNA obtained was determined on a spectrophotometer (Cecil, USA) or in ethidium bromide stained 1% agarose gel.
PCR amplification for quality check and HPV detection
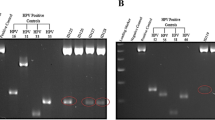
PCR based approach was used for detection for high- risk types HPV16/18 and low- risk types HPV6/11 DNA were carried out as described previously20,21 using type-specific primers: HPV16 (1), 5′ -AAG GCC AAC TAA ATG TCA C-3′; HPV16 (2), 5′ -CTG CTT TTA TAC TAA CCG G-3′; HPV18 (1), 5′ -ACC TTA ATG AAA AAC CAC GA-3′; HPV18 (2), 5′ -CGT CGT TTA GAG TCG TTC CTG-3′. Initially, all DNA samples were tested for β-globin gene amplification using a pair of primers: β-globin (1), 5′ -GAA GAG CCA AGG ACA GGT AC-3′; β-globin (2), 5′ -CAA CTT CAT CCA CGT TAC ACC-3′ and the presence of HPV by using a pair of consensus primers located within the conserved L1 open reading frame (ORF) of the HPV genome (MY 11, 5 -GCM CAG GGW CAT AAY AAT GC-3; MY 09, 5 -CGT CCM ARR GGA WAC TGA TC-3; where M A C, WA T, Y CT, R A G). PCR was performed in a 25 μl reaction mix containing 50–100 ng DNA, 10 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 125 μM of each dNTP (dATP, dCTP, dGTP and dTTP), 5 pmol of each oligonucleotide primer and 0.3 U Taq DNA polymerase (Bangalore Genei). The temperature profile used for amplification constituted an initial denaturation at 95°C for 5 min followed by 35 cycles with denaturation at 95°C for 30 sec, annealing at 55–57°C for 30 sec and extension at 72°C for 1 min, which was extended for 7 min in the final cycle. After amplification, 15 μl aliquot of PCR products were electrophoresed and visualized on an ethidium bromide-stained 2–3% Nusieve agarose gel under a short wave UV transilluminator and gel documented on Alpha digi doc (Alpha Digi Doc, Bio Rad).
Statistical analysis
Statistical analysis on the data was performed using Epi-info version 6.0 software (Center of Disease Control and Prevention, Atlanta, USA). Fischer's exact test, chi square test were used where ever applicable. These tests were considered statistically significant when p ≤ 0.05.
Results
Prostate tissue biopsies and their clinical-pathological characteristics
In the present study, a total of one hundred and fifty (n = 150) histopathologically confirmed, prostate biopsies comprising 55 Benign prostate hyperplasia (BPH) that served as controls and 95 invasive cancers were collected. The various clinical and demographic characteristics of prostate carcinoma cases and BPH controls and the status of their HPV infection is presented in Table 1.
Most of prostate cancer cases were in the age group of 60–69 years while that of BPH controls were <60 years. The PSA level was found to be in the range of 10–20 ng/ml in 29% (28/95) cancer patients compared to 7% (4/55) in BPH controls while higher PSA level (>20 ng/ml) was measured in 10% (10/95) cases and none in BPH controls. The carcinoma of the prostate was pathologically confirmed in all the cases and the Gleason score (highly differentiated, score ≤6; moderately differentiated, score 7; poorly differentiated, score ≥8) was evaluated by pathologist using the Gleason scoring system22,23. Patient stratification with respect to Gleason score revealed that 50% (48/95), 13% (12/95) and 37% (35/95) of the cases had a Gleason score of ≤6, 7 and ≥8 respectively (Table 1). Clinically 24% (23/95) of prostate cancer cases had late presentation either stage III or IV while 76% (72/95) belonged to stage I or II (Table 1).
Prevalence of Human Papillomavirus infection in Prostate Cancer
Human papilloma virus infection has been found to be one of the co-factor associated with the progression/development of prostate cancer, tissue samples were screened by PCR amplification for both high-risk HPV type 16/18 and low risk type 6/11 infection initially using a pair of L1 consensus sequence primers MY09/MY11. The data revealed HPV infection in 41% (39/95) of tumor biopsies and 20% (11/55) in benign hyperplasia (BPH) that served as disease controls.
Subsequent PCR- based HPV typing using type - specific primers revealed that out of 39 HPV L1 positive tumors, 77% (30/39) were infected with HPV type 16 and 15% (6/39) were found to be positive for HPV type 18 and rest three cases were infected with either HPV type 6 (5%, 2/39) or HPV type 11 (2%, 1/39) Table 2 and 3.
While out of 11 L1 BPH positive cases, majority (7/11, 64%) of the BPH controls were infected with low risk type 6 (6/7) and type 11 (1/7) and only fraction of samples (4/11, 36%) were infected with either high risk type 16/18. None of the HPV infected tumors either cancer/BPH showed any co- infection with other high- risk/low-risk HPV types (Table 3).
In order to determine the cofactors which may increase the risk of HPV infection in prostate cancer; association of HPV infection with various demographic and clinical characteristics of the patients was examined (Table 3). Significant proportion of HPV infected (74%) cases belonged to stage III and IV (p < 0.001) with most of them belonging to high Gleason score ≥8 (p = 0.003). Majority (60%) of the cases with a Gleason score ≥8 were significantly infected with HPV16 (p = 0.006) compared to 9%, 3% and 3% with HPV18, HPV6 and HPV11 infection respectively (Table 3). In contrast, no significant association of HPV infection could be coupled with age and mean PSA level of the patients (Table 3).
Discussion
The oncogenic potential of high risk HPVs and their role as a vital etiologic factor in the development of cancer is well documented13. The present scenario of various molecular approaches in prostate cancer is focusing on the controversial role of HPV infection in the prostate carcinogenesis16,18,24,25. Though contrasting studies have reported the presence of HPV and other viral infections in prostate cancer worldwide7,25, none of the studies till date have been reported from India. Therefore, the present study was designed to study the HPV infection status in prostate cancer cases and BPH controls from North Indian population. Previous findings have given sufficient evidences that oncogenic subtypes of HPV, with the most common types 16 and 18, have a strong association with anogenital and other epithelial cancers13,21,24,26,27,28,29,30,31. However, the role of HPV in prostate carcinogenesis is still debatable and needs to be elucidated. Previous studies that showed immortalization of prostate epithelial cells with HPV-16 or 18 DNA implicated for the positive association between HPV infection and prostate cancer18,32. The present study demonstrated that a significant number of prostate cancer cases were infected with HPV accounting for about 41% of the total cases and 20% in BPH controls. This is in concordance with previous and recent epidemiological studies carried out world over that showed presence of HPV in as high as 65% of prostate tumors and established association of certain specific HPV types as a risk factor in causing of progression to prostate cancer17,18,33,34. Leiros et al detected HPV in 41.5% (17/41) in prostate carcinoma samples from Argentina35 which is similar to the findings of the present study. Our results are also in agreement with Zambrano et al that showed HPV infection in 41% of prostate tumor cases in California6. In another study HPV infection was observed in 65.3% of prostate cancer and 48% in BPH controls36.
Further stratification of the results revealed that a significantly high proportion (77%) of HPV positive cases was infected with high risk type HPV 16. A previous study has also reported high-risk HPV type 16 positivity in 53.8% cancer and in 20.0% benign biopsies36. The analysis of low risk HPV types 6 and 11 showed that BPH controls harbored a higher proportion of low risk HPV types (13%) as compared to only 3% in prostate cancer. This finding reflects the important role of HPV low risk types in formation of benign and precancerous lesions in prostate cancer as HPV6 and 11 have been well accepted to form precancerous lesions during cervical cancer13. In contrast certain studies from different geographical regions have also reported almost nil HPV infection in prostate cancer37,38. These discrepancies in the status of HPV infection worldwide indicate the influence of demographic factors like geographical area, ethnicity, lifestyle in the prevalence of HPV infection in prostate cancer.
In the present study a highly significant correlation was found between HPV infection and prostate carcinoma stage. Though HPV infection was more prevalent in advanced stage of cancer with a high gleason score, the entry of HPV in prostate cancer appears to be at the BPH stage as 20% of these showed HPV infection Therefore, there is a possibility that HPV may be significantly influencing both disease initiation and disease progression during prostate carcinogenesis. No significant association of HPV infection was found with respect to PSA level and mean age of patients.
Therefore, the present study strengthens the hypothesis that the prostate gland in males represents a complex niche where multiple infections with oncogenic DNA viruses like HPV occur and implicates the potential role of these viruses in progression of prostate cancer. To our knowledge, this is the pioneering study evaluating the frequency of HPV infection in prostate cancer in India. The study guarantees the clinical relevance of HPV infection in prostate carcinogenesis that has been underestimated till date in Indian population.
References
Tyagi, B., Manoharan, N. & Raina, V. A case control study on prostate cancer in Delhi. Asian Pac J Cancer Prev 11, 397–401 (2010).
Yeole, B. B. Trends in the prostate cancer incidence in India. Asian Pac J Cancer Prev 9, 141–4 (2008).
Center, M. M. et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 61, 1079–92 (2012).
Taylor, M. L., Mainous, A. G. 3rd & Wells, B. J. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam Med 37, 506–12 (2005).
Shavers, V. L., Underwood, W. & Moser, R. P. Race/ethnicity and the perception of the risk of developing prostate cancer. Am J Prev Med 37, 64–7 (2009).
Zambrano, A., Kalantari, M., Simoneau, A., Jensen, J. L. & Villarreal, L. P. Detection of human polyomaviruses and papillomaviruses in prostatic tissue reveals the prostate as a habitat for multiple viral infections. Prostate 53, 263–76 (2002).
Fioriti, D. et al. A case of human polyomavirus Bk infection in a patient affected by late stage prostate cancer: could viral infection be correlated with cancer progression? Int J Immunopathol Pharmacol 20, 405–11 (2007).
Kundu, S. D. et al. The toll-like receptor pathway: a novel mechanism of infection-induced carcinogenesis of prostate epithelial cells. Prostate 68, 223–9 (2008).
Sutcliffe, S. et al. Prostate involvement during sexually transmitted infections as measured by prostate-specific antigen concentration. Br J Cancer 105, 602–5 (2011).
Wu, J. J., Huang, D. B., Pang, K. R. & Tyring, S. K. Cutaneous metastasis to the chest wall from prostate cancer. Int J Dermatol 45, 946–8 (2006).
Milde-Langosch, K., Riethdorf, S. & Loning, T. Association of human papillomavirus infection with carcinoma of the cervix uteri and its precursor lesions: theoretical and practical implications. Virchows Arch 437, 227–33 (2000).
McMurray, H. R., Nguyen, D., Westbrook, T. F. & McAnce, D. J. Biology of human papillomaviruses. Int J Exp Pathol 82, 15–33 (2001).
Das, B. C., Hussain, S., Nasare, V. & Bharadwaj, M. Prospects and prejudices of human papillomavirus vaccines in India. Vaccine 26, 2669–79 (2008).
Henderson, B. E. & Feigelson, H. S. Hormonal carcinogenesis. Carcinogenesis 21, 427–33 (2000).
Adami, H. O., Kuper, H., Andersson, S. O., Bergstrom, R. & Dillner, J. Prostate cancer risk and serologic evidence of human papilloma virus infection: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 12, 872–5 (2003).
Aghakhani, A. et al. The role of human papillomavirus infection in prostate carcinoma. Scand J Infect Dis 43, 64–9 (2011).
Chen, A. C. et al. Human papillomavirus in benign prostatic hyperplasia and prostatic adenocarcinoma patients. Pathol Oncol Res 17, 613–7 (2011).
Lin, Y. et al. Human papillomavirus 16 or 18 infection and prostate cancer risk: a meta-analysis. Ir J Med Sci 180, 497–503 (2011).
Nayyar, R., Sharma, N. & Gupta, N. P. Prognostic factors affecting progression and survival in metastatic prostate cancer. Urol Int 84, 159–63 (2010).
Singh, N. et al. Downregulation of tumor suppressor gene PML in uterine cervical carcinogenesis: impact of human papillomavirus infection (HPV). Gynecol Oncol 128, 420–6 (2013).
Sobti, R. C. et al. Aberrant promoter methylation and loss of suppressor of cytokine signalling-1 gene expression in the development of uterine cervical carcinogenesis. Cell Oncol (Dordr) 34, 533–43 (2011).
Epstein, J. I., Allsbrook, W. C. Jr., Amin, M. B. & Egevad, L. L. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol 29, 1228–42 (2005).
Egevad, L., Srigley, J. R. & Delahunt, B. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens: rationale and organization. Mod Pathol 24, 1–5 (2011).
Al Moustafa, A. E. Involvement of human papillomavirus infections in prostate cancer progression. Med Hypotheses 71, 209–11 (2008).
Gazzaz, F. S. & Mosli, H. A. Lack of detection of human papillomavirus infection by hybridization test in prostatic biopsies. Saudi Med J 30, 633–7 (2009).
Hussain, S. et al. Transcription factor AP-1 in esophageal squamous cell carcinoma: alterations in activity and expression during human Papillomavirus infection. BMC Cancer 9, 329 (2009).
Hussain, S. et al. Methylation-mediated gene silencing of suppressor of cytokine signaling-1 (SOCS-1) gene in esophageal squamous cell carcinoma patients of Kashmir valley. J Recept Signal Transduct Res 31, 147–56 (2011).
Thakur, N. et al. Genetic variant of CCND1: association with HPV-mediated cervical cancer in Indian population. Biomarkers 14, 219–25 (2009).
Singh, N. et al. Overexpression of signal transducer and activator of transcription (STAT-3 and STAT-5) transcription factors and alteration of suppressor of cytokine signaling (SOCS-1) protein in prostate cancer. J Recept Signal Transduct Res 32, 321–7 (2012).
Sobti, R. C. et al. Deregulation of STAT-5 isoforms in the development of HPV-mediated cervical carcinogenesis. J Recept Signal Transduct Res 30, 178–88 (2010).
Sobti, R. C. et al. Overexpression of STAT3 in HPV-mediated cervical cancer in a north Indian population. Mol Cell Biochem 330, 193–9 (2009).
Naghashfar, Z., DiPaolo, J. A., Woodworth, C. D. & Passaniti, A. Immortalization of human adult prostatic adenocarcinoma cells by human papilloma virus HPV16 and −18 DNA. Cancer Lett 100, 47–54 (1996).
Anwar, K. et al. Presence of ras oncogene mutations and human papillomavirus DNA in human prostate carcinomas. Cancer Res 52, 5991–6 (1992).
Gherdovich, S. et al. [Detection of the human papillomavirus in hyperplastic and cancerous prostatic tissue with PCR]. Minerva Urol Nefrol 49, 73–7 (1997).
Leiros, G. J. et al. Detection of human papillomavirus DNA and p53 codon 72 polymorphism in prostate carcinomas of patients from Argentina. BMC Urol 5, 15 (2005).
Carozzi, F. et al. Association of human papillomavirus with prostate cancer: analysis of a consecutive series of prostate biopsies. Int J Biol Markers 19, 257–61 (2004).
Silvestre, R. V. et al. Low frequency of human papillomavirus detection in prostate tissue from individuals from Northern Brazil. Mem Inst Oswaldo Cruz 104, 665–7 (2009).
Tu, H., Jacobs, S. C., Mergner, W. J. & Kyprianou, N. Rare incidence of human papillomavirus types 16 and 18 in primary and metastatic human prostate cancer. Urology 44, 726–31 (1994).
Author information
Authors and Affiliations
Contributions
N.S. conceived the study, collected the samples and carried out all the experiments and primary manuscript writing. S.H. critically reviewed the manuscript. N.K. senior pathologist who did the histopathological analysis of the prostate specimens. S.K. professor in urology who provided clinical samples and revision of manuscript. R.C. and M.B. senior scientists who contributed to critical revision of the manuscript, supervised and guaranteed the work. All authors read and approved the final manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Singh, N., Hussain, S., Kakkar, N. et al. Implication of high risk Human papillomavirus HR-HPV infection in prostate cancer in Indian population- A pioneering case-control analysis. Sci Rep 5, 7822 (2015). https://doi.org/10.1038/srep07822
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07822
This article is cited by
-
Human papillomavirus and prostate cancer: systematic review and meta-analysis
Scientific Reports (2023)
-
A matched case-control study in Taiwan to evaluate potential risk factors for prostate cancer
Scientific Reports (2023)
-
Multiple pathogens and prostate cancer
Infectious Agents and Cancer (2022)
-
Modifiable risk factors for prostate cancer in low- and lower-middle-income countries: a systematic review and meta-analysis
Prostate Cancer and Prostatic Diseases (2022)
-
Evidence for a causal role by human papillomaviruses in prostate cancer – a systematic review
Infectious Agents and Cancer (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.