Abstract
Globally, cervical cancer, whose etiologic factor is Human papillomavirus (HPV), is the third most common cancer among women. In cervical cancer screening, HPV testing is important. However, the prevalence of HPV in northern Guangdong Province has not been conclusively determined. A total of 100,994 women attending Yuebei People's Hospital Affiliated to Shantou University Medical College between 2012 and 2020 were recruited. HPV was tested by a polymerase chain reaction (PCR)-based hybridization gene chip assay. The prevalence of HPV among these women was established to be19.04%. Peak prevalence was observed in women aged 40–49 (7.29%). Besides, the prevalence of single-type HPV infection (14.46%) was significantly high, compared to multiple-type infection (4.58%) (p < 0.01), while the prevalence of high-risk HPV infection (19.97%) was significantly higher than that of low-risk genotypes (5.48%) (p < 0.01). The most prevalent high-risk genotypes were HPV52 (4.16%), HPV16 (2.98%), HPV58 (2.15%), HPV53 (1.58%) and HPV68 (1.34%). HPV co-infection with up to 10 genotypes was reported for the first time. Our findings suggested a high burden of HPV infections among women in northern Guangdong. Establishing the prevalence and genotype distribution characteristics of HPV infections in the region can contribute to cervical cancer prevention through HPV vaccination.
Similar content being viewed by others
Introduction
Globally, cervical cancer which can be caused by the major oncogenic types of HPV, is the third most common cancer in women1,2. Therefore, HPV vaccination can significantly reduce the risk of cervical cancer3. HPV is a circular double-stranded DNA virus that mainly infects epithelial cells in the skin and mucosal cells. The International Agency for Research on Cancer (IARC) reports that there are more than 200 HPV genotypes, among which, about 40 genotypes infect the genitourinary system while 20 genotypes are associated with cancer development4. Most HPV infections have no obvious clinical symptoms, and the spontaneously resolve5. Based on their risk of causing cancer, HPVs are classified as high-risk HPV (HR-HPV) or low-risk HPV (LR-HPV). HR-HPV can cause cervical cancer, with HPV 16/18 exhibiting the highest risk of cancer development. It usually takes 10–20 years for HPV infection-driven lesions to progress to malignancy6,7,8.
There are regional differences in HPV prevalence and genotype distribution, which explains the different types of vaccines9,10. The currently licensed HPV vaccines include bivalent vaccines (HPV16/18), quadrivalent vaccines (HPV16/18/6/11), andnine-valent vaccines (HPV16/18/6/11/31/33/45/52/58)11,12. Therefore, to select a suitable vaccine, it is important to understand local HPV incidences and subtype distribution.
This study aimed to investigate the prevalence and genotype distribution of HPV in northern Guangdong province, so as to provide a scientific basis for early screening strategies and vaccination for cervical cancer in the region.
Materials and methods
Subjects
A total of 100,994 women attending the Physical Examination Center and Department of Gynecology of Yuebei People’s Hospital between January 1st, 2012 and December 31st, 2020 were enrolled in this study. The inclusion criteria were: (1) Aged 18–75 years; (2) All participants resided in northern Guangdong Province; (3) No history of sexual life or vaginal medication in the past one week; (4) Willingness to undergo an HPV test and participate in the present study, All participants signed informed consent. This research was approved by the Ethics Committees of Yuebei People’s Hospital (KY-2019-083, Approved October 10, 2019). All methods were performed in accordance with relevant guidelines and standard operating procedures (SOPs).
Specimen collection
Gynecological practitioners collected the cervical specimens using cervical brush. First, the cervix was exposed using a vaginal dilator, secretions were wiped using a cotton swab, the cervical brush was extended into the cervix and rotated 4 to 5 times in a clockwise direction to obtain a sufficient amount of cervical epithelial cells. Then, the brush was placed in a vial containing the cell preservation solution (Yaneng Biotechnology, Ltd., Shenzhen, China). Cervical samples should be stored at room temperature for no more than 12 h and at 4 °C for no more than 7 days after collection. Specimens were sent to the department of clinical PCR laboratory in the hospital for testing.
DNA extraction
DNA from exfoliated cervical cells was extracted using a DNA extraction kit (Yaneng Biotechnology, Ltd., Shenzhen, China). Samples on the cervical brushes were eluted; the elution was transferred to a 1.5 ml centrifuge tube, centrifuged at 13,000 rpm for 10 min, and the supernatant discarded. To the cell pellet, 50 µl of the lysis buffer was added to suspend the pellet, incubated at 100 °C for 10 min, and centrifuged at 13,000 rpm for 10 min, to obtain DNA.
HPV DNA testing
HPV DNA PCR amplification and genotyping were performed using the HPV genotyping Kit (Yaneng Biotechnology, Ltd., Shenzhen, China) according to the manufacturer’s instructions. The test kit was approved by the National Medical Products Administration (NMPA, No.20193401918). The test included 23 type-specific oligonucleotides, designed to detect 17 HR-HPV genotypes (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82) together with 6 LR-HPV genotypes (HPV6, 11, 42, 43, 81 and 83). Amplification was performed using the BIOER Genetouch PCR amplification instrument. The amplification system consisted of 5 μl HPV-DNA and 20 μl reaction system. Reaction conditions were: 50 °C for 15 min, denaturation at 95 °C for 10 min, denaturation at 94 °C for 30 s, annealing at 42 °C for 90 s, elongation at 72 °C for 30 s, 40 cycles, elongation at 72 °C for 5 min, and storage at 4 °C. HPV genotyping was performed by hybridization in a YN-H16 thermostatic hybridization instrument. The final result was determined by directly observing color reactions on the chip. The negative control showed no color reaction at all sites except the blue dot on the internal control (IC) site. The positive control must be color reaction of the positive HPV genotype and IC site (shown in blue), and the genotype is read according to the site of the blue spot on the chip.
Statistical analysis
Data analysis was performed using SPSS22.0 software (IBM, USA). Data were presented as mean ± standard deviation or frequency and percentage for numerical or discrete variables, respectively. The Chi-square test was used to determine significant differences between groups, and differences were considered statistically significant at p < 0.05.
Ethics statement
This research was approved by the Ethics Committees of Yuebei People’s Hospital.
Results
Age-specific HPV prevalence
A total of 100,994 women visited the Yuebei People's Hospital and subjected to HPV DNA testing. The basic characteristics of study participants are shown in Table 1. It was established that 19,233 (19.04%) of the women had HPV infections, with most of the infections occurring in women of the 40–49 age group (7.29%). Single-type HPV infections were common among women aged 40–49 years (6.44%), while multiple-type HPV infections were common among women aged 50–59 years (1.71%). Single-type HPV infections (14.46%) were more common than multiple-type infections (4.58%), (p < 0.01; Table 2 and Fig. 1).
HPV genotype distribution
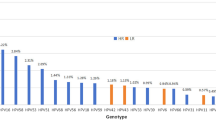
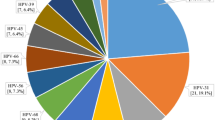
A total of 25,710 positive HPV genotypes were detected in samples from the 100,994 women (Since some women were infected with multiple genotypes, so the total number of positive HPV genotypes was greater than the number of infected women). Twenty-three HPV genotypes were identified (Table 3 and Fig. 2). The proportions of HR-HPV and LR-HPV infections were 19.97% and 5.48%, respectively. The top ten most prevalent HR-HPV genotypes were HPV52, 16, 58, 53, 68, 51, 18, 56, 33, and 59, with corresponding proportions of 4.16%, 2.98%, 2.15%, 1.58%, 1.34%, 1.28%, 1.10%, 1.03%, 0.89% and 0.77%, respectively (Fig. 3). The LR-HPV genotypes included HPV81, 43, 42, 6, 11 and 83, with corresponding proportions of 1.98%, 1.29%, 0.91%, 0.74%, 0.41% and 0.15%, respectively (Fig. 4). The distribution of HR-HPV in high-grade lesions cervical and invasive cervical cancer is shown in Table 4.
Distribution of single and multiple HPV infections
Single, double, and multiple-type HPV infections accounted for 75.94%, 17.52% and 4.47%, respectively (Table 5). Single-type infection was more common than multiple-type infection (p < 0.01). HPV co-infection with up to 10 subtypes was discovered for the first time (Fig. 5).
Discussion
In this study, we established that the HPV infection rate among women in northern Guangdong was 19.04%, with the infection rate being highest among women aged 40–49 (7.29%). Single-type and HR-HPV infections were the most prevalent form. The most prevalent HR-HPV infections were HPV52, 16, 58, 53, 68, 51, 18, and 56 while LR-HPV infections were mainly HPV81 and 43. We also report, for the first time, HPV co-infection with up to 10 genotypes in two cases.
Studies have reported differences in HPV prevalence and genotype distribution in different countries13. According to a comprehensive meta-analysis involving over 1 million women, the global HPV prevalence is estimated to be 11.7%. Sub-Saharan Africa (24.0%), Eastern Europe (21.4%), and Latin America (16.1%) have the highest prevalence14. In Japan, the prevalence of HPV is 25%, with HPV52, 16, and 58 being the most common genotypes15. In Korea, the prevalence of HPV is 16.71%, with HPV 53, 58, and 52 being the most common genotypes16. A previous meta-analysis reported that HPV infection rate among women in mainland China is 19.0%, with HPV16, 52, and58 being the most common genotypes17. These types are also the most common types of CIN in China18. This contrasts with the situation in Western countries, where HPV16 and 18 infections are prevalent19,20.
In China, there are regional differences in HPV prevalence and genotype distribution. Population-based screening revealed that the HPV infection rate in China ranges from 9.9% to 31.94%21,22,23,24. Among the sampled regions, Haikou (31.94%), Beijing (21.06%), and Guangdong (20.02%) have the highest HPV incidences. Our results are consistent with HPV distribution characteristics previously reported in China25,26,27. The overall HPV infection rate is high and single-type as well as high-risk infections are more common. However, the top five genotypes are slightly different. For instance, the main genotypes in Meizhou28, were HPV 16, 52, 58, 18, and 81, while in northern Guangdong, they were HPV 52, 16, 58, 53 and 68. In short, the top three in most regions of China were HPV 52, 16, and 58.
HPV screening and vaccination are the most effective measures for preventing cervical cancer. China's cervical cancer screening program adopts a combined method for HPV and cytology (TCT) screening, which can maximize screening Sensitivity and specificity to improve the detection rate. The program is led by the government, with the participation of social groups, medical institutions, third-party testing institutions, etc. It is completely free for the subjects, voluntary, and covers women aged 35–64. In short, cervical cancer screening has a long way to go, and various efforts are being made to explore solutions with Chinese characteristics. Most developed countries have adopted vaccination as their main preventive strategy. Therefore, if these prevention strategies can be promoted in developing countries, thousands of lives can be saved. Currently, the three available HPV vaccines are all prepared according to the existing epidemiological characteristics of HPV in Western countries. At the same time, there is controversy with regards the vaccination age. The American Cancer Society (ACS) recommends that HPV vaccination be performed at 9 to 12 years old to reduce cervical cancer incidences. However, ACS does not endorse the 2019 Advisory Committee on Immunization Practices recommendation for shared clinical decision for adults aged 27 to 45 years potential of vaccination29.
China approved the importation of bivalent vaccines in 2016. Nationally, the HPV vaccination rate for women is only 11.0%30, and the vaccination situation is not optimistic. HPV vaccination is expensive, and uncertainties regarding the side effects of the vaccine are the main reason for reluctance. In this study, we found that the HPV infection rate remained stable without any downward trend from 2012 to 2020. Therefore, China needs to develop HPV vaccines that are suitable for the Chinese population, while putting into consideration the characteristics of vaccine genotypes and the age of vaccination, further localization, reducing costs, and including government free vaccination programs to reduce the incidences of HPV infections and cervical cancer.
Conclusions
For the first time, we report on prevalence and subtype distribution of women HPV in northern Guangdong, which is of great significance for guiding the formulation of local vaccination and other prevention strategies. However, epidemiological investigation of HPV requires multi-center and long-term monitoring, especially evaluation of the effects after vaccination. Besides, persistent HR-HPV and multiple-type infections as well as cancer outcomes should be investigated further.
Data availability
The original data can be obtained by contacting the corresponding author.
References
Arbyn, M. et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob. Health. 8, e191–e203. https://doi.org/10.1016/S2214-109X(19)30482-6 (2020).
Crosbie, E. J., Einstein, M. H., Franceschi, S. & Kitchener, H. C. Human papillomavirus and cervical cancer. Lancet 382, 889–899. https://doi.org/10.1016/S0140-6736(13)60022-7 (2013).
Lei, J. et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 383, 1340–1348. https://doi.org/10.1056/NEJMoa1917338 (2020).
World Health Organization. Human papillomavirus vaccines: WHO position paper. May 2017-Recommendations. Vaccine 35, 5753–5755. https://doi.org/10.1016/j.vaccine.2017.05.069 (2017).
Kreisel, K. M. et al. Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2018. Sex Transm Dis. 48, 208–214. https://doi.org/10.1097/OLQ.0000000000001355 (2021).
Burd, E. M. Human papillomavirus laboratory testing: The changing paradigm. Clin. Microbiol. Rev. 29, 291–319. https://doi.org/10.1128/CMR.00013-15 (2016).
Bharti, A. C., Singh, T., Bhat, A., Pande, D. & Jadli, M. Therapeutic startegies for human papillomavirus infection and associated cancers. Front. Biosci. (Elite Ed). 10, 15–73. https://doi.org/10.2741/e808 (2018).
Doorbar, J., Egawa, N., Griffin, H., Kranjec, C. & Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 25, 2–23. https://doi.org/10.1002/rmv.1822 (2015).
de Sanjosé, S. et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: A meta-analysis. Lancet Infect. Dis. 7, 453–459. https://doi.org/10.1016/S1473-3099(07)70158-5 (2007).
Krul, E. J. et al. Human papillomavirus in malignant cervical lesions in Surinam, a high-risk country, compared to the Netherlands, a low-risk country. Int J Gynecol Cancer. 9, 206–211. https://doi.org/10.1046/j.1525-1438.1999.99020.x (1999).
Patel, H., Jeve, Y. B., Sherman, S. M. & Moss, E. L. Knowledge of human papillomavirus and the human papillomavirus vaccine in European adolescents: A systematic review. Sex Transm. Infect. 92, 474–479. https://doi.org/10.1136/sextrans-2015-052341 (2016).
Thanasas, I., Lavranos, G., Gkogkou, P. & Paraskevis, D. Understanding of young adolescents about HPV infection: How health education can improve vaccination rate. J. Cancer Educ. 35, 850–859. https://doi.org/10.1007/s13187-019-01681-5 (2020).
Yan, X. et al. Prevalence of human papillomavirus infection and type distribution among Uyghur females in Xinjiang, northwest China. Oncol. Lett. 20, 25. https://doi.org/10.3892/ol.2020.11886 (2020).
Bruni, L. et al. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 202, 1789–1799. https://doi.org/10.1086/657321 (2010).
Sasagawa, T., Maehama, T., Ideta, K., Irie, T., Fujiko Itoh J-HERS Study Group. Population-based study for human papillomavirus (HPV) infection in young women in Japan: A multicenter study by the Japanese human papillomavirus disease education research survey group (J-HERS). J. Med. Virol. 88, 324–335. https://doi.org/10.1002/jmv.24323 (2016).
Ouh, Y. T. et al. Prevalence of human papillomavirus genotypes and precancerous cervical lesions in a screening population in the Republic of Korea, 2014–2016. J. Gynecol. Oncol. 29, e14. https://doi.org/10.3802/jgo.2018.29.e14 (2018).
Li, K., Li, Q., Song, L., Wang, D. & Yin, R. The distribution and prevalence of human papillomavirus in women in mainland China. Cancer 125, 1030–1037. https://doi.org/10.1002/cncr.32003 (2019).
Zhang, J., Cheng, K. & Wang, Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch. Gynecol. Obstet. 302, 1329–1337. https://doi.org/10.1007/s00404-020-05787-w (2020).
Rogovskaya, S. I. et al. Human papillomavirus prevalence and type-distribution, cervical cancer screening practices and current status of vaccination implementation in Russian Federation, the Western countries of the former Soviet Union, Caucasus region and Central Asia. Vaccine 31, H46-58. https://doi.org/10.1016/j.vaccine.2013.06.043 (2013).
Cohen, P. A., Jhingran, A., Oaknin, A. & Denny, L. Cervical cancer. Lancet 393, 169–182. https://doi.org/10.1016/S0140-6736(18)32470-X (2019).
Zeng, Z. et al. Prevalence and genotype distribution of HPV infection in China: Analysis of 51,345 HPV genotyping results from China’s largest CAP certified laboratory. J. Cancer 7, 1037–1043. https://doi.org/10.7150/jca.14971 (2016).
Jiang, L. et al. HPV prevalence and genotype distribution among women in Shandong Province, China: Analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. PLoS ONE 14, e0210311. https://doi.org/10.1371/journal.pone.0210311.eCollection2019 (2019).
Wang, R. et al. Nationwide prevalence of human papillomavirus infection and viral genotype distribution in 37 cities in China. BMC Infect. Dis. 15, 257. https://doi.org/10.1186/s12879-015-0998-5 (2015).
Ma, L. et al. Characteristics of women infected with human papillomavirus in a tertiary hospital in Beijing China, 2014–2018. BMC Infect. Dis. 19, 670. https://doi.org/10.1186/s12879-019-4313-8 (2019).
Guo, C. et al. The prevalence and distribution of human papillomavirus among 10,867 Chinese Han women. Infect. Agent Cancer 16, 21. https://doi.org/10.1186/s13027-021-00360-9 (2021).
Yuan, X. W., Li, Y. J., Qiu, Q., Luo, Z. Y. & Zhao, X. F. Prevalence and genotype distribution of human papillomavirus among 9945 women from the Nanhai area of Foshan. BMC Infect. Dis. 19, 71. https://doi.org/10.1186/s12879-019-3687-y (2019).
Zhong, T. Y. et al. Prevalence of human papillomavirus infection among 71,435 women in Jiangxi Province, China. J. Infect. Public Health 10, 783–788. https://doi.org/10.1016/j.jiph.2017.01.011 (2017).
Zhao, P. et al. Prevalence and genotype distribution of human papillomavirus infection among women in northeastern Guangdong Province of China. BMC Infect. Dis. 18, 204. https://doi.org/10.1186/s12879-018-3105-x (2018).
Saslow, D. et al. Human papillomavirus vaccination 2020 guideline update: American Cancer Society guideline adaptation. CA Cancer J. Clin. 70, 274–280. https://doi.org/10.3322/caac.21616 (2020).
You, D. et al. Human papillomavirus (HPV) vaccine uptake and the willingness to receive the HPV vaccination among female college students in china: a multicenter study. Vaccines 8, 31. https://doi.org/10.3390/vaccines8010031 (2020).
Funding
This work was supported by the Science and Technology Fund of Guangdong (210128166270630), the Science and Technology Innovation Strategy Project of Guangdong of China (201803011) and the Health Research Project of Shaoguan (Y20172).
Author information
Authors and Affiliations
Contributions
W.H., H.X., H.H., and Z.M. contributed to conception and design of the study. D.Z., Y.L., Y.G., F.X. and W.C. organized the database. W.H. and Z.M. wrote the first draft of the manuscript. All authors contributed to manuscript revision, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, W., Xu, H., Hu, H. et al. The prevalence of human papillomavirus among women in northern Guangdong Province of China. Sci Rep 12, 13353 (2022). https://doi.org/10.1038/s41598-022-17632-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-17632-y
This article is cited by
-
Prevalence of human papillomavirus infection and associated factors among women attending cervical cancer screening in setting of Addis Ababa, Ethiopia
Scientific Reports (2024)
-
Prevalence and risk factors of oral human papillomavirus infection among 4212 healthy adults in Hebei, China
BMC Infectious Diseases (2023)
-
The prevalence of HPV among 164,137 women in China exhibited some unique epidemiological characteristics
Infectious Agents and Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.