Abstract
The repetition of experience is often necessary to establish long-lasting memory. However, the cellular mechanisms underlying this repetition-dependent consolidation of memory remain unclear. We previously observed in organotypic slice cultures of the rodent hippocampus that repeated inductions of long-term potentiation (LTP) led to a slowly developing long-lasting synaptic enhancement coupled with synaptogenesis. We also reported that repeated inductions of long-term depression (LTD) produced a long-lasting synaptic suppression coupled with synapse elimination. We proposed these phenomena as useful in vitro models for analyzing repetition-dependent consolidation. Here, we hypothesized that the enhancement and suppression are mediated by the brain-derived neurotrophic factor (BDNF)-TrkB signaling pathway and the proBDNF-p75NTR pathway, respectively. When we masked the respective pathways, reversals of the enhancement and suppression resulted. These results suggest the alternative activation of the p75NTR pathway by BDNF under TrkB-masking conditions and of the TrkB pathway by proBDNF under p75NTR-masking conditions, thus supporting the aforementioned hypothesis.
Similar content being viewed by others
Introduction
We know from our own daily life that the establishment of long-lasting memory or learning often requires the repetition of experience. In behavioral experiments in animals, it is a standard protocol to apply repeated training or task performance in order to establish learning in either vertebrates1,2 or invertebrates3,4. However, the cellular mechanisms that underlie this repetition-dependent consolidation of memory remain unclear.
In organotypic slice cultures of the rodent hippocampus, we previously demonstrated that 3 repeated inductions of long-term potentiation (LTP) by chemical means led to a slowly developing (requiring ~ 1 week for full development) and long-lasting (lasting more than 2 weeks after its development) enhancement of synaptic transmission in the CA3-CA1 synapses and this was accompanied by the formation of new synaptic structures5,6. We named this novel structural plasticity phenomenon repetitive-LTP-induced synaptic enhancement (RISE).
In contrast, when we induced long-term depression (LTD, a phenomenon that is apparently symmetrical to LTP) 3 times by chemical means, a slowly developing and long-lasting synaptic suppression that was apparently symmetrical to RISE was provoked, which was coupled to the elimination of existing synapses7,8. We called this phenomenon LTD-repetition-operated synaptic suppression (LOSS). We propose that RISE and LOSS are in vitro model phenomena appropriate for cell biological analyses of the repetition-dependent consolidation of memory. In addition, we hypothesized that these 2 opposite forms of structural plasticity are mediated by brain-derived neurotrophic factor (BDNF) and its precursor proBDNF.
BDNF is a 119-amino-acid-long basic polypeptide and has been long known as an antiapoptotic protein, a promoter of neurite extension in developing nervous systems and an inducer of synapse formation in developed brain9,10,11,12. BDNF is synthesized in its precursor form (proBDNF), which has a preceding acidic polypeptide composed of 110 amino acids at the N-terminus that is cleaved later by processing proteases to generate mature BDNF (mBDNF). Recently, proBDNF has been shown to have its own biological effects that are quite opposite to mBDNF. It acts as a proapoptotic factor and a neurite extension suppressor in the developing brain and a synapse elimination inducer in the developed brain. These symmetrical activities of mBDNF and proBDNF are called the yin-yang effect of this neurotrophin13,14,15. In relation to RISE and LOSS, a RISE-producing stimulus increases the levels of expression of BDNF mRNA and protein16 and a LOSS-producing stimulus increases the cellular content of proBDNF17. mBDNF is known to bind to receptor tyrosine kinase TrkB as a high-affinity receptor and to p75 neuroptophin receptor (p75NTR) as a low-affinity receptor18,19. proBDNF primarily binds to p75NTR, but it can also bind to TrkB13,20.
From these facts, we hypothesized that RISE is produced through activation of the mBDNF-TrkB signaling pathway, whereas LOSS is produced through activation of the proBDNF-p75NTR signaling pathway17. If this is true, it is logically expected that a RISE-producing stimulus should produce LOSS when TrkB is masked and a LOSS-producing stimulus should produce RISE when p75NTR is masked. In the present study, we conducted these experiments to test our hypothesis.
Results
In this study, we used organotypic slice cultures of the mouse hippocampus instead of those of the rat hippocampus used previously. The reasons for this choice were to demonstrate that RISE and LOSS are not species-specific phenomena and to prepare for the wider use of transgenic animals. As expected, mouse cultures showed equivalent structural plasticity phenomena as those that have been shown previously in rat cultures5 (see also Supplementary Fig. S1 on line).
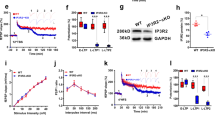
Mature form of BDNF (mBDNF) has long been known as an inducer of synapse formation11. In the present mouse slice culture, the application of mBDNF for 4 days produced RISE-equivalent synaptic strength enhancement that was coupled with an increase in synapse number when assayed 14 days later (Fig. 1a–c). We have previously shown16 that a RISE-producing stimulus (3 repeated inductions of LTP) results in increased levels of expression of the mRNA and protein of BDNF, suggesting the involvement of BDNF in the development of RISE. In fact, the application of a BDNF scavenger, which is a chimeric molecule of TrkB and IgG-Fc21, inhibited the establishment of RISE after the third induction of chemical LTD (Fig. 1d–f). mBDNF exerts its effects by activating its high-affinity receptor TrkB18. As expected, the application of a function-blocking antibody against TrkB22 after the third LTP induction inhibited the establishment of RISE (Fig. 2).
Possible involvement of BDNF signaling in the long-lasting synaptic enhancement induced by repetitive inductions of LTP.
(a–c) Increases in synaptic strength and spine density produced by a 4-day application of mature BDNF (mBDNF). (a) Timeline of the experimental procedures. The day of BDNF application, which corresponded to 13–15 days in vitro, is reckoned as poststimulus (PS) day 0. The electrical and morphometric assays were performed on PS days 14–21. (b) The synaptic strength, as indicated by the maximal fEPSP amplitude (see Methods for explanation), is shown together with representative recordings. The numbers of slices examined were 16 for control (mock), 10 for BDNF and 14 for BDNF + TrkB-Fc. The scale bars are 10 msec (horizontal) and 1 mV (vertical). (c) The dendritic spine density is shown together with representative images of dendritic segments. The numbers of segments [slices] examined were 43 [13] for control, 24 [9] for BDNF and 41 [12] for BDNF + TrkB-Fc. The scale bar indicates 2 μm. (d–f) Increases in synaptic strength and spine density produced by 3 repeated inductions of chemical LTP and inhibition by TrkB-Fc. (d) Timeline of the experimental procedures. TrkB-Fc, a BDNF scavenger, is applied for 3 days beginning 1 hour after the third LTP induction. (e) The synaptic strength is shown together with representative recordings. The numbers of slices examined were 18 for control (3 × mock), 19 for 3 × forskolin (FK) and 12 for 3 × FK + TrkB-Fc. The scale bars are 10 msec (horizontal) and 1 mV (vertical). (f) The dendritic spine density is shown together with representative images of dendritic segments. The numbers of dendritic segments [slices] are 31 [6] for control, 47 [11] for 3 × FK and 26 [8] for 3 × FK + TrkB-Fc. The scale bar indicates 2 μm. Statistical significance is indicated by asterisks: The P values are 0.018 for *1, 0.046 for *2, 7.28 × 10−5 for ***3, 2.34 × 10−5 for ***4, 1.77 × 10−6 for ***5, 8.37 × 10−6 for ***6, 1.88 × 10−9 for ***7 and 1.24 × 10−5 for ***8.
The anti-TrkB antibody inhibits the long-lasting synaptic enhancement induced by repetitive inductions of LTP.
(a) Timeline of the experimental procedures. The anti-TrkB antibody was applied for 3 days after 3 × FK applications beginning 1 hour after the third LTP induction. (b) The synaptic strength, as indicated by the maximal fEPSP amplitude, is shown together with representative recordings. The numbers of slices examined are 14 for control (3 × mock), 13 for 3 × FK and 8 for 3 × FK + anti-TrkB. The scale bars are 10 msec (horizontal) and 1 mV (vertical). (c) The dendritic spine density is shown together with representative images of the dendritic segments. The numbers of segments [slices] examined are 25 [5] for control, 24 [7] for 3 × FK and 24 [6] for 3 × FK + TrkB-Fc. The scale bar indicates 2 μm. The P values are 2.63 × 10−5 for ***1, 0.0066 for **2, 8.48 × 1 0−6 for ***3 and 1.10 × 10−5 for ***4.
Here, we needed to be cautious about the possibility that the BDNF scavenger might interfere with the induction of LTP, because, if it occurred, the LTP would be induced only twice. In acute hippocampal slice preparations, the BDNF scavenger actually inhibited the maintenance of LTP23,24,25 and we confirmed this (see Supplementary Fig. S2 on line). Keeping this in mind, in the experiments shown in Fig. 1 and Fig. 2, we began the application of the BDNF scavenger after waiting 60 min following the withdrawal of forskolin (FK) in order to allow the maintenance of LTP for at least 80 min. We suspected that 80 min of LTP was still insufficient for the development of RISE; therefore, we sometimes waited 3 hours before applying the BDNF scavenger and the results were the same (see Supplementary Fig. S3 on line).
The fact that the BDNF scavenger affected the FK-induced LTP in the present specimens suggested that the release of endogenous mBDNF accompanied each of the 3 LTP inductions. This led to the question of what would be the result when TrkB was masked upon all 3 LTP inductions. As shown in Fig. 3, such prolonged masking of TrkB by a function-blocking antibody resulted not in a simple inhibition of RISE establishment but instead in an apparent reversal of RISE to LOSS. This result was readily explained by the alternative activations of p75NTR by mBDNF when TrkB was masked chronically. In support of this explanation, neither RISE nor LOSS resulted when both TrkB and p75NTR (by a function-blocking antibody26,27 of p75NTR) were masked.
Prolonged application of the anti-TrkB antibody during repetitive inductions of LTP leads to a reversal of synaptic enhancement.
(a) Timeline of the experimental procedures. (b) The synaptic strength, as indicated by the maximal fEPSP amplitude, is shown together with representative recordings. The numbers of slices examined are 19 for control (3 × mock), 13 for 3 × FK, 22 for 3 × FK + anti-TrkB and 14 for 3 × FK + anti-TrkB + anti-p75NTR. The scale bars are 10 msec (horizontal) and 1 mV (vertical). (c) The dendritic spine density is shown together with representative images of the dendritic segments. The numbers of segments [slices] examined are 41 [12] for control, 37 [11] for 3 × FK, 47 [13] for 3 × FK + anti-TrkB and 45 [11] for 3 × FK + anti-TrkB + anti-p75NTR. The scale bar indicates 2 μm. The P values are 4.09 × 10−5 for ***1, 0.0016 for **2, 3.81 × 10−11 for ***3, 8.34 × 10−5 for ***4, 6.72 × 10−3 for **5, 0.039 for *6, 8.21 × 10−5 for ***7, 2.52 × 10−11 for ***8 and 0.0061 for **9 and 0.0010 for **10.
Thereon we performed a symmetrical examination: what would be the result if a LOSS-producing stimulus (3 repeated inductions of chemical LTD) was given when p75NTR was masked during all 3 LTD inductions. As shown in Fig. 4a–c, the prolonged masking of p75NTR by the antip75NTR antibody did not result in a simple inhibition of LOSS establishment but instead resulted in an apparent reversal from LOSS to RISE. This result was explained by the alternative activations of TrkB by proBDNF when p75NTR was masked chronically. When both TrkB and p75NTR were masked, the LOSS-producing stimulus produced neither RISE nor LOSS.
Prolonged application of the anti-p75NTR antibody during repetitive inductions of LTD leads to a reversal of synaptic suppression.
(a) Timeline of the experimental procedures. (b) The synaptic strength, as indicated by the maximal fEPSP, is shown together with representative recordings. The numbers of slices examined are 16 for control (3 × mock), 17 for 3 × DHPG, 8 for 3 × DHPG + anti-p75NTR and 13 for 3 × DHPG + anti-p75NTR + anti-TrkB. The scale bars are 10 msec (horizontal) and 1 mV (vertical). (c) The dendritic spine density is shown together with representative images of the dendritic segments. The numbers of segments [slices] examined are 38 [8] for control, 36 [9] for 3 × DHPG, 20 [5] for 3 × DHPG + anti-p75NTR and 40 [9] for 3 × DHPG + anti-p75NTR + anti-TrkB. The scale bar indicates 2 μm. (d, e) The immunoblotting analysis, supporting the alternative activation of TrkB by proBDNF under p75NTR-masking conditions. Lysates were prepared 3 hours after the third LTD induction. Five slices were pooled for preparing 1 sample (d). Two bands that are immunopositive to the anti-TrkB antibody are full-length (FL-) and truncated (Trunc-) forms of TrkB47. The bands are taken out from a single membrane. (For original images of the membrane, see Supplementary Fig. S4 on line). The quantified levels of phosphorylated Trk, total full-length TrkB and p75NTR are shown in (e). The analysis was repeated 8 times (4 times for p75NTR), the density of each band was relativized to that of control (3 × mock) for each time and the mean values are plotted here. The P values are 0.016 for *1, 7.12 × 10−4 for ***2, 1.00 × 10−7 for ***3, 0.019 for *4, 0.0015 for **5, 0.025 for *6, 0.0029 for **7, 1.66 × 10−7 for ***8, 0.0087 for **9, 0.0063 for **10 and 0.0032 for **11.
The alternative TrkB activation assumed here was confirmed by an immunoblotting analysis (Fig. 4d, e) in which significant phosphorylation of TrkB was recognized. The expression levels of TrkB and p75NTR proteins were not affected significantly by the antibody treatment.
Confirmation of the alternative activation of p75NTR, when a RISE-producing stimulus was given under TrkB-masking conditions, was desired but it was not conducted, since the downstream signals of p75NTR are still undetermined.
Discussion
The aim of the present study was to examine our hypothesis that the two apparently symmetrical long-lasting structural plasticity phenomena, RISE5 and LOSS7, are mediated by the yin-yang effects of mBDNF and proBDNF.
There have been many reports demonstrating that mBDNF increases synaptic strength and promotes synapse formation23,28,29. There are accumulating lines of evidence that proBDNF lowers synaptic strength and drives synapse elimination30,31. In slice cultures of the rodent hippocampus, exogenously applied mBDNF and proBDNF produce synapse formation and elimination, respectively (Fig. 1 of this report and Fig. 4 of Egashira et al.17). However, these results do not necessary prove that endogenous mBDNF and proBDNF mediate RISE and LOSS. Thus, we applied a BDNF scavenger here to neutralize the endogenous neurotrophin.
Although it is desired to quantify directly the absolute amounts of BDNF and proBDNF released (setting aside their cellular contents) to prove their involvement in RISE and LOSS, such quantification is still difficult, since released BDNF is quickly bound to TrkB, p75NTR and the extracellular matrices32 and proBDNF is rapidly converted to BDNF15. So we chose a strategy to use function-blocking antibodies against endogenous neurotrophin receptors.
BDNF binds to TrkB with high affinity and transmits neurotrophic signals in the cell through multiple pathways; however, it also binds to p75NTR with low affinity11. proBDNF binds to p75NTR and transmits antineurotrophic signals through an intracellular pathway unspecified yet14 and it also binds to TrkB20. Hence, when their proper receptors are masked, mBDNF and proBDNF should bind alternatively to their substitute receptors in order to transmit inverse signals into the cell. The present results demonstrated that this actually occurs, thus, providing support for the aforementioned hypothesis.
It is possible that TrkB masking might have transformed LTP to LTD. If so, the 3 repeated LTP inductions would have been 3 repeated inductions of LTD, which should eventually lead to LOSS. However, Lu et al.33 as well as Fig. S2 of this report indicate that TrkB masking interferes with the maintenance of LTP but does not cause LTD. In TrkB-knockout mice, LTP becomes short-lasting; but it is not transformed to LTD34. The possibility that the p75NTR-masking may have transformed LTD to LTP should also be low because the knockout of p75NTR diminished LTD but did not reverse LTD to LTP31.
It is a matter of argument whether masking of neurotrophin receptors by antibodies is possible. In literature, the binding affinity of BDNF to p75NTR is ~ 10−9 M in dissociation constant (Kd), whereas that to TrkB is ~ 10−11 M (ref. 19). Assuming the Kd of anti-TrkB antibody's binding to TrkB 10−10 M, the most common value of antibody binding to antigen, BDNF could defeat the antibody in the competition of binding to TrkB. However, this high binding affinity of BDNF to TrkB is realized through its concomitant binding to p75NTR and the binding of BDNF to TrkB solely is ~ 10−9 M in Kd, which is similar to its binding to p75NTR (ref. 35). This situation is equivalent to the case of NGF's binding to TrkA and p75NTR (ref. 36). Therefore, it is likely that anti-TrkB antibody defeats BDNF in the competition of binding to TrkB and that BDNF alternatively binds to p75NTR. The same argument is applicable to the masking of p75NTR by anti-p75NTR antibody. The Kd value of proBDNF and p75NTR is not found in literature, but applying the value of IC50 (~10−8 M) obtained by neutralization experiment of proBDNF by a p75NTR-Fc scavenger14, the masking of p75NTR by the anti-p75NTR antibody is expectable.
For the inhibition of biological effects by antibodies, we have to take the numbers of molecules of ligands, receptors and antibodies into account, in addition to the issues of affinities. However, we presently did not estimate them because of technical difficulties mentioned above. It is also difficult to estimate the penetration of antibodies into the tissue. Thus, we cannot calculate to which proportions the TrkB and p75NTR molecules were masked actually. Nevertheless, in our previous study17, the biological effects of both endogenous proBDNF and exogenously applied cleavage-resistant proBDNF (100 ng/mL, equivalent to 3.8 nM on a monomer basis) were inhibited by the anti-p75NTR antibody at a dose identical to that we use here. Tanaka et al.22 applied anti-TrkB antibody at a dose similar to ours to the cultured rat hippocampal slices and effectively inhibited the biological effect of BDNF.
The alternative activation of TrkB under the p75NTR-masking conditions was demonstrated by detecting phosphorylated TrkB (Fig. 4). The antibody used here to detect phospho-TrkB also recognizes phospho-TrkA and phospho-TrkC. Hence, the increased level of phospho-Trk may not represent the increase level of phospho-TrkB. But with considering that a RISE-producing stimulus was nullified by a BDNF-scavenger (Fig. 1), it is unlikely that a LOSS-producing stimulus under p75NTR-masking condition activates TrkA or TrkC instead of TrkB to bring about a phenomenon equivalent to RISE.
Now that we have obtained supports to the hypothesis that RISE and LOSS are mediated by the mBDNF-TrkB and proBDNF-p75NTR signaling pathways, respectively, we next need to test how cells use mBDNF and proBDNF differentially, because both molecules are products of the same gene. Transcriptional or translational control seems impossible. The site of conversion (i.e., limited proteolysis by some processing proteases) from proBDNF to mBDNF is under debate30,37. If the conversion occurs intracellularly, we have to assume that there is a differential exocytotic mechanism between mBDNF-containing (i.e., protease-possessing) vesicles and proBDNF-containing (i.e., protease-lacking) vesicles. If the conversion occurs after exocytosis, we can assume that there is a mechanism that regulates extracellular protease activity. Recently, a new possibility has been presented, which states that the conversion occurs intracellularly but the excised propeptide co-exists in equimolar amounts within the vesicle without further decomposition38. The propeptide (which is an acidic polypeptide) binds to mBDNF (which is a basic polypeptide) to reconstruct a proBDNF-equivalent complex (M. Kojima, personal communication). If this is the case, there must be a mechanism modifying the electrostatic interactions between those polypeptides.
RISE in slice cultures of the rodent hippocampus has the following properties: 3 repetitions of LTP induction are necessary and sufficient5; the protein kinase A-dependent phase of LTP is mandatory6, the repetition must have intervals of 3–24 hours5,6; RISE is not an extension of LTP, since it develops after the third LTP disappears5,6; it occurs input pathway-specifically as shown by pathway bisection and electric tetanization onto one of the pathways39; RISE is accompanied by expression of a set of cytoskeleton-regulating proteins16; the increase in the number of dendritic spines is the result of increased fluctuation followed by a biased fluctuation40; the spines do not divide but bud newly from dendritic shaft40,41; an equivalent synapse formation is reproducible in dissociated hippocampal cell cultures42,43; an opposite long-lasting structural plasticity, or LOSS, is produced after repeated inductions of LTD7,8,17. In addition to these phenomenological aspects, with the possible contribution of BDNF and proBDNF to RISE and LOSS shown here, these phenomena provide a mechanistic clue to the repetition-dependent consolidation of memory.
Methods
Organotypic slice culture of the mouse hippocampus
Neonates of C57BL/6J mice (Japan SLC, Inc., Hamamatsu, Japan) of postnatal days 6–7 (both sexes) were sacrificed after anesthetization. The hippocampi were isolated and 400-μm-thick slices were cut perpendicularly to the long axis of the tissue from the middle 1/3 portion with a Mcllwain tissue chopper. Four or 5 slices were placed on top of a polytetrafluoroethylene membrane (Millicell-CM, EMD Millipore Corporation, Billerica, MA, USA) and submerged in culture medium that was composed of 50% minimal essential medium based on Hanks' salts, 25% Hanks' buffered saline and 25% heat-inactivated house serum (all from Life Technologies Corporation, Grand Island, NY, USA). The cultures were maintained at 34°C in a humidified atmosphere for 13–15 days until maturation5,41 with the medium renewed every 3–4 days.
Chemical induction of LTP and LTD
To maintain the cultures aseptically for 2–3 weeks after the LTP/LTD inductions, we adopted chemical means to induce LTP/LTD. LTP was induced by FK (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), an adenylyl cyclase activator and thus an inducer of late phase-LTP, according to conventional protocol5. The stimulation medium containing 20 μM FK was prepared immediately before use. The 13–15 days in vitro culture was exposed to the stimulation medium and kept at 34°C for 20 min, which was followed by washing with a serum-free medium and bathing in a fresh culture medium. For the control groups, the culture was treated the same way except for the inclusion of FK in the stimulation medium.
LTD was induced by the application of 3,5-dihydroxyphenylglycine (DHPG; Tocris Bioscience, Bristol, UK), an agonist of group-I metabotropic glutamatergic receptors, according to a conventional protocol17. The culture was exposed to 50 μM of DHPG dissolved in a balanced salt solution (in mM: 149 NaCl, 5 KCl, 2 MgSO4, 2.5 CaCl2, 10 glucose, 10 HEPES-NaOH, pH 7.3) and kept at 34°C for 10 min, which was followed by washing with a serum-free medium and bathing in a fresh culture medium.
The same LTP- and LTD-inducing procedures were repeated 3 times at 24-hour intervals to produce RISE and LOSS, respectively. The day of the first induction of LTP/LTD was reckoned as poststimulus (PS) day 0.
The following drugs and chemicals were applied after or concomitantly with the LTP/LTD induction(s): anti-TrkB antibody (1:1,000; 610101; BD Biosciences, Franklin Lakes, NJ, USA), anti-p75NTR antibody (1:1,000; AB1554; EMD Millipore Corporation), BDNF (100 ng/mL; Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) and TrkB-Fc (1 μg/mL; Sigma-Aldrich Co., St. Louis, MO, USA). The onsets and durations of the drug applications are described in the text and are shown diagrammatically in the timeline in each figure.
Electrophysiology
The culture was transferred to a recording chamber perfused with artificial cerebrospinal fluid (ACSF; composed of [in mM]: 126 NaCl, 5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 2.0 MgSO4, 22 NaHCO3, 10 glucose). The ACSF was bubbled with a gas mixture (95% O2/5% CO2) to maintain the pH at 7.3 and perfused at 1.0 mL/min. A tungsten monopolar electrode was placed at the CA3 pyramidal cell layer and test stimuli were delivered every 30 sec. Synaptic responses were recorded with a glass microelectrode filled with ACSF with an input resistance of 1–5 MΩ and placed in the CA1 pyramidal cell layer.
We followed the standard protocol for estimating synaptic strength in cultured slices5. In studies that use acute slices, usually the slope of field excitatory postsynaptic potentials (fEPSP) recorded from the dendritic layer is monitored. In the case of cultured slices, however, the dendritic fEPSP slopes vary markedly among the specimens, presumably because of the imperfect myelination of input axons. The comparison by fEPSP slopes assumes a priori the coherent onset of EPSPs in the population of neurons under examination. However, this does not hold in a cultured slice. The fEPSP slope recorded in a cultured slice does not represent a sum of the slopes of individual EPSPs, instead a degree of coherence in the onset of EPSPs evoked by inputs arriving nonuniformly in time. Therefore, as Muller et al.44 have emphasized, the fEPSP amplitude, rather than the slope, is a better measure of the synaptic strength in cultured slices. In addition, because we needed to know the total synaptic input to the CA1 neurons that represents the number of synapses possessed by the neurons, we applied supramaximal stimulation (usually 0.3 mA) to activate practically all functional input fibers and recorded fEPSPs that were integrated at the somatic layer. By recording those maximal fEPSPs from at least 3 sites in the CA1 region for each culture, we adopted the largest value of the maximal fEPSP amplitudes as a representative value of the synaptic strength for that culture.
Lucifer yellow injections
Intracellular injections of fluorescent dye were conducted following the methods of Pace et al.45 with slight modifications. The cultured slice was fixed mildly with 2% paraformaldehyde in a balanced salt solution and a glass micropipette filled with 8% Lucifer yellow Li salt (LY; Nacalai Tesque, Inc., Kyoto, Japan) was inserted slowly into the CA1 pyramidal cell layer. Penetration of the micropipette into the pyramidal cell was recognizable by the sudden emergence of cell-shaped fluorescence. A negative current (1–3 nA) was applied for 3–5 min through the micropipette. The LY injection was performed on 1 or 2 cells for each slice. The slice was then fixed overnight with 4% paraformaldehyde.
Quantification of spine density
The slices that were injected with LY were imaged by a laser scanning confocal microscope (FV-300; Olympus Corporation, Tokyo, Japan) equipped with a water immersion objective lens (UplanSApo 60X, NA 1.20). The Z-axis step was 0.5 μm. A dendritic segment of a secondary (or occasionally tertiary) branch of an apical dendrite located 100 μm or less from the soma and ran horizontally for 10–30 μm was subjected to spine number (density) counting. To identify the spines, we followed the criterion of Holtmaat et al.46. The number of spines was divided by the segment length to obtain the density.
Immunoblotting analysis
Five cultured slices of the same treatment group were removed from the polytetrafluoroethylene membrane filter and lysed with an ice-cold lysis buffer (composed of [in mM] 20 Tris HCl [pH 7.4], 300 NaCl, 1 EDTA, 10 NaF, 10 sodium pyrophosphate, 0.1 phenylarsine oxide, 1% Triton X-100, 1% phosphatase inhibitor [Sigma-Aldrich Co.] and 1% protease inhibitor [Roche Diagnostics GmbH, Mannheim, Germany]). The lysate was centrifuged at 15,000 rpm for 10 min at 4°C and the supernatant was boiled at 100°C for 10 min. The samples were loaded onto 10% SDS gels and separated by applying a constant voltage of 200 V for 35 min and then the proteins were transferred onto a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a constant voltage of 60 V for 4 hours. After blocking with a blocking buffer (composed of 2% bovine serum albumin and 0.05% Tween-20) for 30 min, the membranes were washed with Tris-buffered saline with Tween-20 (TBS-T; composed of 150 mM NaCl, 100 mM Tris and 0.1% Tween-20 [pH 7.2]) and incubated with primary antibodies overnight at 4°C. The primary antibodies used were anti-phosphorylated Trk (9141S; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-TrkB (1:1,000; 610101; BD Biosciences), anti-p75NTR (1:2000; AB1554; EMD Millipore) and anti-TUJ1 (1:3,000; Covance, Inc., Princeton, NJ, USA) diluted in TBS-T. The membranes were washed with TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies (1:10,000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 30 min at room temperature. After washing with TBS-T, a luminol-based chemiluminescence assay kit (Chemi-Lumi One Super; Nacalai Tesque) for the horseradish peroxidase reaction was used and the membranes were exposed to radiographic film (RX-U; Fuji Film, Tokyo, Japan). The images of the protein bands were quantified with ImageJ software. The band density was normalized to that of TUJ1.
Statistical analysis
All experiments were repeated using cultures prepared from more than 2 litters. The numbers of examined specimens are indicated in the figure legends. All data are presented as mean ± standard error of means. For statistical comparisons between groups, at first, the normality of the data was assessed using χ2 tests for goodness of fit and then the homogeneity of variance was assessed using Bartlett's test. Because all of the data, except for the data from the immunoblotting experiment (Fig. 4d, e), showed normal distributions and non-different variances, they were further analyzed by one-way ANOVA followed by a Tukey-Kramer test. The data for the immunoblotting experiment (Fig. 4e) were analyzed by a Steel-Dwass test. The levels of significance are indicated by the asterisks: * for P < 0.05, ** for P < 0.01 and *** for P < 0.001 in all the figures.
Animal care
This study was conducted in accordance with the Regulation on Animal Experiments of the Animal Experiments Committee of Osaka University. The protocols were approved by the Committee for Animal Experiments of our School (No. 12-027).
References
Whishaw, I. Q. & Kolb, B. The Behavior of the Laboratory Rat: A Handbook with Tests. [520pp] (Oxford Univ. Press, New York, 2004).
Gerfen, C. R., Rogawski, M. A., Sibrey, D. R., Skolnick, P. & Wray, S. (eds.) Short Protocols in Neuroscience: Systems and Behavioral Methods. [610pp] (John Wiley, Hoboken, 2006).
Connolly, J. B. & Tully, J. Behavior, learning and memory. Chapter 9 in Drosophila: A Practical Approach. 2nd Ed. [Roberts D. B. (ed.)] [416pp] (Oxford Univ. Press, New York, 1998).
Schacher, S., Kandel, E. R. & Montarolo, P. cAMP and arachidonic acid simulate long-term structural and functional changes produced by neurotransmitters in Aplysia sensory neurons. Neuron 10, 1079–1088 (1993).
Tominaga-Yoshino, K., Kondo, S., Tamotsu, S. & Ogura, A. Repetitive activation of protein kinase A induces slow and persistent potentiation associated with synaptogenesis in cultured hippocampus. Neurosci. Res. 44, 357–367 (2002).
Tominaga-Yoshino, K., Urakubo, T., Okada, M., Matsuda, H. & Ogura, A. Repetitive induction of late-phase LTP produces long-lasting synaptic enhancement accompanied by synaptogenesis in cultured hippocampal slices. Hippocampus 18, 281–293 (2008).
Shinoda, Y., Kamikubo, Y., Egashira, Y., Tominaga-Yoshino, K. & Ogura, A. Repetition of mGluR-dependent LTD causes slowly developing persistent reduction in synaptic strength accompanied by synapse elimination. Brain Res. 1042, 99–107 (2005).
Kamikubo, Y. et al. Long-lasting synaptic loss after repeated induction of LTD: independence to the means of LTD induction. Eur. J. Neurosci. 24, 1606–1616 (2006).
Barde, Y. A., Edgar, D. & Thoenen, H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1, 549–553 (1982).
Kalcheim, C., Barde, Y. A., Thoenen, H. & Le Douarin, N. M. In vivo effect of brain-derived neurotrophic factor on the survival of developing dorsal root ganglion cells. EMBO J. 6, 2871–2873 (1987).
McAllister, A. M., Katz, L. C. & Lo, D. C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 22, 295–318 (1999).
Huang, E. J. & Reichardt, L. F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736 (2001).
Lee, R., Kermani, P., Teng, K. K. & Hempstead, B. L. Regulation of cell survival by secreted proneurotrophins. Science 294, 1945–1948 (2001).
Teng, H. K. et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 25, 5455–5463 (2005).
Lu, B., Pang, P. T. & Woo, N. H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 6, 603–614 (2005).
Kawaai, K. et al. Analysis of gene expression changes associated with long-lasting synaptic enhancement in hippocampal slice cultures after repetitive exposures to glutamate. J. Neurosci. Res. 88, 2911–2922 (2010).
Egashira, Y. et al. Involvement of the p75NTR signaling pathway in persistent synaptic suppression coupled with synapse elimination following repeated longterm depression induction. J. Neurosci. Res. 88, 3433–3446 (2010).
Klein, R. et al. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell 66, 395–403 (1991).
Rodriguez-Tébar, A., Dechant, G. & Barde, Y. A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron 4, 487–92 (1990).
Fayard, B., Loeffler, S., Weis, J., Vöglein, E. & Krüttgen, A. The secreted brain-derived neurotrophic factor precursor pro-BDNF binds to TrkB and p75NTR but not to TrkA or TrkC. J. Neurosci. Res. 80, 18–28 (2005).
Ninkina, N., Grashchuck, M., Buchman, V. L. & Davis, A. M. TrkB variants with deletions in the leucine-rich motifs of the extracellular domain. J. Biol. Chem. 272, 13019–13025 (1997).
Tanaka, J. et al. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science 319, 1683–1687 (2008).
Figurov, A., Pozzo-Miller, L. D., Olafsson, P., Wang, T. & Lu, B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381, 706–709 (1996).
Kang, H., Welcher, A. A., Shelton, D. & Schuman, E. M. Neurotrophins and time: Different roles for TrkB signaling in hippocampal long-term potentiation. Neuron 19, 653–664 (1997).
Patterson, S. L. et al. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phosphor-MAP kinase. Neuron 32, 123–140 (2001).
Gehler, S., Gallo, G., Veien, E. & Letourneau, P. C. p75 neurotrophin receptor signaling regulates growth cone filopodial dynamics through RhoA activity. J. Neurosci. 24, 4363–4372 (2004).
Pehar, M. et al. Modulation of p75NTR-dependent motor neuron death by a small non-peptidyl mimetic of the neurotrophin loop 1 domain. Eur. J. Neurosci. 24, 1575–1580 (2006).
Tyler, W. & Pozzo-Miller, L. D. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J. Neurosci. 21, 4249–4258 (2001).
Alonso, M., Medina, J. H. & Pozzo-Miller, L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn. Mem. 11, 172–178 (2004).
Pang, P. T. et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306, 487–491 (2004).
Woo, N. H. et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat. Neurosci. 8, 1069–1077 (2005).
Hartmann, D., Drummond, J., Handberg, E., Ewell, S. & Pozzo-Miller, L. Multiple approaches to investigate the transport and activity-dependent release of BDNF and their application in neurogenetic disorders. Neural Plast. 2012, 203734 (2012).
Lu, Y. et al. TrkB as a potential synaptic and behavioral tag. J. Neurosci. 31, 11762–11771 (2011).
Minichiello, L. et al. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron 36, 121–137 (2002).
Barker, P. A. & Shooter, E. M. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron 13, 203–15 (1994).
Bibel, M., Hoppe, E. & Barde, Y. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 18, 616–22 (1999).
Matsumoto, T. et al. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 11, 131–133 (2008).
Dieni, S. et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 196, 775–788 (2012).
Oe, Y., Tominaga-Yoshino, K. & Ogura, A. Local establishment of repetitive long-term potentiation-induced synaptic enhancement in cultured hippocampal slices with divided input pathways. J. Neurosci. Res. 89, 1419–1430 (2011).
Oe, Y., Tominaga-Yoshino, K., Hasegawa, S. & Ogura, A. Dendritic spine dynamics in synaptogenesis after repeated LTP inductions: Dependence on pre-existing spine density. Sci. Rep. 3, 1957; 10.1038/srep01957 (2013).
Urakubo, T., Tominaga-Yoshino, K. & Ogura, A. Ultrastructural features of hippocampal CA1 synapses with respect to synaptic enhancement following repeated PKA activation. Neurosci. Lett. 407, 1–5 (2006).
Yamamoto, M., Urakubo, Y., Tominaga-Yoshino, K. & Ogura, A. Long-lasting synapse formation in cultured hippocampal neurons after repeated PKA activation. Brain Res. 1042, 6–16 (2005).
Taniguchi, N. et al. Possible involvement of BDNF release in long-lasting synapse formation induced by repetitive PKA activation. Neurosci. Lett. 406, 38–42 (2006).
Muller, D., Buchs, P. A. & Stoppini, L. Time course of synaptic development in hippocampal organotypic cultures. Brain Res. 71, 93–100 (1993).
Pace, C. J., Tieman, D. G. & Tieman, S. B. Intracellular injection in fixed slices: obtaining complete dendritic arbors of large cells. J. Neurosci. Methods 15, 23–30 (2002).
Holtmaat, A. et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat. Protoc. 4, 1128–1144 (2009).
Beck, K. D. et al. Induction of noncatalytic TrkB neurotrophin receptors during axonal sprouting in the adult hippocampus. J. Neurosci. 13, 4001–4014 (1993).
Acknowledgements
We are grateful to Dr. Masami Kojima (National Institute of Advanced Industrial Science and Technology of Japan) for communication and discussion. This study was supported by grants-in-aid from the Japan Science and Technology Agency to A.O. (23300132 and 24650207).
Author information
Authors and Affiliations
Contributions
K.T.-Y. and A.O. designed research; S.S. performed research (electrophysiology, morphology and biochemistry); K.T.-Y. performed research (pilot electrophysiology); and A.O. wrote the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareALike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Sakuragi, S., Tominaga-Yoshino, K. & Ogura, A. Involvement of TrkB- and p75NTR-signaling pathways in two contrasting forms of long-lasting synaptic plasticity. Sci Rep 3, 3185 (2013). https://doi.org/10.1038/srep03185
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep03185
This article is cited by
-
BDNF and Lactate as Modulators of Hippocampal CA3 Network Physiology
Cellular and Molecular Neurobiology (2023)
-
The evolution of BDNF is defined by strict purifying selection and prodomain spatial coevolution, but what does it mean for human brain disease?
Translational Psychiatry (2022)
-
Long-term depression at hippocampal mossy fiber-CA3 synapses involves BDNF but is not mediated by p75NTR signaling
Scientific Reports (2021)
-
Early Downregulation of p75NTR by Genetic and Pharmacological Approaches Delays the Onset of Motor Deficits and Striatal Dysfunction in Huntington’s Disease Mice
Molecular Neurobiology (2019)
-
The ProNGF/p75NTR pathway induces tau pathology and is a therapeutic target for FTLD-tau
Molecular Psychiatry (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.