Abstract
Introduction:
We report a case of acute tetraplegia, without any trauma or symptoms prior to onset, who presented with ossification of the posterior longitudinal ligament (OPLL) in the cervical spine with concomitant spinal cord infarction.
Case Presentation:
A 64-year-old man with a number of risk factors for vascular disease was admitted to our hospital with progressive motor weakness in the bilateral upper and lower extremities. He had initially felt numbness in his left upper extremity and had no previous neurological symptoms or trauma. The night after the initial symptoms, he developed spastic tetraplegia requiring respiratory support. Computed tomography images of the cervical spine demonstrated the segmental type of OPLL. Spinal cord compression and signal intensity changes were identified at the level of C3/4 on magnetic resonance imaging (MRI). He underwent emergency surgery consisting of posterior decompression with laminoplasty of C3-6. Despite the surgery, the patient’s tetraplegia did not improve and he continued to require respirator support. There was still no improvement in his neurological status at 10 days postoperatively, and MRI demonstrated evidence of marked spinal cord infarction.
Discussion:
Mechanical compression of spinal arteries by OPLL and pre-existing vascular compromise had a role in the pathogenesis of spinal cord infarction. Chronic spinal compression may be characterized by 3 important factors, namely an uncommonly devastating clinical course, vascular risk factors and persistent findings on MRI, and these might lead to early diagnosis of spinal cord infarction.
Similar content being viewed by others
Introduction
Ossification of the posterior longitudinal ligament (OPLL) may contribute to the acute onset or deterioration of cervical myelopathy, occasionally tetraplegia, after minor trauma.1–4 For the patients without myelopathy, only 17–20% developed myelopathy in prospective studies.3,5,6 There is usually only chronic progression even in patients who present with neurological symptoms.3,5–7 Therefore, acute presentation in the absence of trauma is unusual in people with OPLL.
There have been some reports of acute tetraplegia caused by cervical disc herniation without trauma.8–11 Our search of the literature, with ‘OPLL’ and ‘tetraplegia’ or ‘quadriplegia’ as keywords in PubMed and Embase (April 2016), found two reports in the literature of tetraplegia in the absence of obvious trauma in patients with OPLL.12,13 However, these 2 cases had previous neurological symptoms several months before admission.
Here we report a patient with acute tetraplegia, without any trauma or symptoms prior to onset, who presented with OPLL in the cervical spine and concomitant spinal cord infarction. The devastating clinical course, risk factors for vascular disease and persistent changes on magnetic resonance imaging (MRI) were important clues for ruling out myelopathy caused by spinal canal stenosis with OPLL. To the best of our knowledge, this is the first case presenting with spinal cord infarction and coexisting OPLL in the cervical spine.
Case report
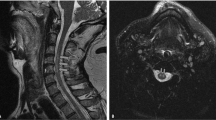
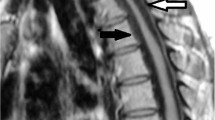
A 64-year-old man was admitted to a previous hospital with progressive motor weakness of the bilateral upper and lower extremities. He had initially felt numbness in his left upper extremity and had no previous neurological symptoms or trauma. Just one night after the initial symptoms, his physical findings had progressed to spastic tetraplegia requiring respiratory support, and he was transferred to our hospital. His past medical history was significant for diabetes mellitus, cerebral infarction, arteriosclerosis obliterans and obesity with a body mass index of 27.1 kg m−2. Sixteen years previously he had undergone anterior cervical discectomy and fusion for cervical disc herniation at C6/7. Neurological examination revealed hypoesthesia below and including the C4 dermatome but intact deep sensation; hyperreflexia in the lower extremities, including bilateral positive Babinski sign; positive Hoffman sign and motor strength of grade 2/5 in the trapezius muscles, grade 1/5 in the deltoid muscles, and grade 0/5 in the biceps muscles and all muscles inferior to them. Computed tomography (CT) demonstrated the segmental type of OPLL between C2 and C6 (Figures 1a and b). Spinal cord compression and signal intensity changes were identified at the level of C3/4 on MRI (Figures 2a and b). The MRI of the brain revealed old infarction involving the left putamen and corona radiate. Although the spinal cord signal intensity changes corresponded to the spinal canal stenosis with OPLL, there was no evidence of trauma that might have acutely precipitated or worsened myelopathy prior to symptom onset. The devastating clinical course suggested a more complicated differential diagnosis, including spinal cord infarction, and we therefore consulted with neurologists and radiologists. Since we could not exclude the possibility of acute deterioration of myelopathy caused by spinal canal stenosis with OPLL, the patient underwent emergency surgery consisting of posterior decompression with C3–6 laminoplasty. Despite the surgery, the patient’s tetraplegia did not improve and he continued to require a respirator. There was still no improvement in his neurological status at 10 days after surgery, at which time MRI demonstrated a large area of spinal cord edema from C2 to C5, with high signal intensity in diffusion-weighted imaging (DWI) and low signal intensity on an apparent diffusion coefficient (ADC) map (Figures 3a–e). The clinical course and persistent MRI findings led to the definitive diagnosis of spinal cord infarction. One month after surgery, the patient was transferred to a rehabilitation hospital. Six months after surgery, his neurological status had not improved and was classified as grade B on the ASIA impairment scale, and he still required use of a respirator.
Magnetic resonance images obtained 10 days after surgery. Sagittal T2-weighted image (a). Axial T2-weighted image (c). And diffusion-weighted image (d). Demonstrate a large area of high signal intensity from C2 to C5, with low signal intensity on the axial T1-weighted (b). Image and the apparent diffusion coefficient map (e).
Discussion
The highest incidence of spinal cord infarction occurs in the thoracolumbar area.14,15 The second most likely to suffer infarction is the mid-cervical area, which may potentially expand to include the phrenic nerve palsy.15 Spinal transient ischemic attacks, prior to the onset of infarction, have been reported in <10% of patients with spinal cord infarction.16,17 Generally, sharp pain and sensory symptoms are noted at the onset of spinal cord ischemia, followed by rapidly progressive motor weakness and potentially complete paralysis within hours.15,18 The current case rapidly progressed to tetraplegia. The devastating clinical course suggested the diagnosis of spinal cord infarction. The presence of spasticity would lead the diagnosis of chronic compression myelopathy rather than spinal cord infarction. However, varying presentation of tendon reflex abnormality can be seen in the literature of acute spinal cord ischemia.19,20 Considering the abnormality of tendon reflex as a long-tract sign, the presentation might be associated with variability in individual blood supply to the spinal cord.19 The presence of spasticity in this patient suggested that the spinal cord might be histologically deformed with degeneration of neurons by chronic spinal cord compression with a long asymptomatic period.21 Moreover, he had a history of prior cerebral infarction with a slight degree of right hemiplegia without subjective symptoms in his activity of daily living. In general, vascular compromise due to factors such as diabetes mellitus, hypertension, hyperlipidemia, peripheral vascular disease, prior cerebral infarction and age over 60 are thought to be involved in the pathogenesis of spinal cord ischemia.18,22 The present case had four vascular risk factors. Millichap et al.22 suggested that, it is important to recognize the vascular risk factor to confirm the diagnosis of spinal cord infarction.
Westwick et al.23 reviewed cases of rapidly progressive myelopathy caused by cervical disc herniation, including four cases with OPLL in the cervical spine. Two of the four patients with OPLL had received cervical massage or manipulation and the remaining two patients had had neck pain for several months prior to the onset of paralysis.23–26 In addition, other investigators reported several cases of acute myelopathy or paralysis caused by cervical disc herniation, which is also rare.8–11 However, there have been no reports of OPLL in the cervical spine causing acute tetraplegia in patients in whom there was no preceding trauma or neurological symptoms, as in the current case. There is no clear evidence whether or not the patient actually presented with cervical myelopathy before the acute presentation. However, the patient was actually asymptomatic before the acute presentation, although he was not aware of any symptoms. In the assessment of previous investigators, the etiology of the spinal cord damage is not only mechanical compression but also spinal ischemia and reperfusion as a secondary spinal cord injury.8–11 Furthermore, Novy et al.16 reported that spinal cord infarction occurred at the level of chronic spinal disease, such as compression fracture, spondylolisthesis, chronic arachnoiditis, and chronic cervical disc protrusion. Yoshizawa27. suggested that chronic compression of the spinal cord induces fibrosis around the lesion, which results in an insufficient blood supply to the spinal cord. Therefore, there is a possibility that OPLL, which causes chronic spinal compression, contributes to the pathology of spinal ischemia. As far as we know, this is the first report of a patient with spinal cord infarction and concomitant OPLL in the cervical spine.
MRI using T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences is useful to detect enlargement of the spinal cord due to ischemia.14 Moreover, it has been noted that signal intensity changes in the spinal cord during the acute ischemic period after infarction might be detected by DWI.28 Reynolds et al.29 reported that spinal cord ischemia resulted in increased DWI signal intensity, with a decreased signal intensity on ADC map images; however, the sensitivity of DWI in the spinal cord has been shown to be lower than in the brain.14 The incidence of signal intensity changes caused by spinal cord ischemia has been reported to range from 45 to 100%, and increases with repeat MRIs.18 Rubin et al.15 suggested that the main purpose of MRI in the acute phase is to identify a compressive lesion, which can be treated with decompression surgery. Indeed, in this case, we identified definitive ischemic changes based on spinal cord intensity changes on MRI images obtained after surgery. When the differential diagnosis is narrowed to spinal cord ischemia, repeat MRI is required. In this case, we did not evaluate the DWI and ADC map of the cervical spine before surgery. The changes of the DWI and ADC map obtained after surgery indicate cytotoxic edema and are consistent with acute ischemic change, which might be irreversible. It cannot be denied that ischemic change might be developed during or after surgery;30–33 however, it is very rare. In addition, we could not see any remarkable difference in his neurological status before and after surgery. Based on clinical course, we are convinced that the spinal cord ischemia occurred before surgery.
In the present case, surgery did not improve the patient’s neurological symptoms. The neurological prognosis after decompression surgery for rapidly progressive myelopathy with cervical disc herniation has ranged from no improvement to full recovery.23 The discrepancy in surgical outcomes may be related to the timing of surgery and the extent of the lesion affected by direct compression or ischemia.11 It is reasonable to assume that surgical decompression would help avoid the spinal cord compression resulting from ischemia-reperfusion edema of the spinal cord.9,11 In order to raise the spinal cord perfusion pressure, combination therapy with hemodynamic augmentation and cerebral fluid drainage has been suggested.15 In contrast with cerebral and myocardial infarction, there is no consensus regarding the optimal anticoagulation therapy for spinal cord infarction and no treatment guidelines for its management.18
Conclusion
The pathogenesis of spinal cord infarction involves mechanical compression of spinal arteries by OPLL and vascular compromise. Spinal cord infarction rarely coexists with a compressive disease, and it is difficult to make a definitive diagnosis in the early stages. Chronic spinal compression may be characterized by three important factors, namely an uncommonly devastating clinical course, vascular risks and persistent changes on MRI, and these might lead to early diagnosis of spinal cord infarction.
References
Chikuda H, Seichi A, Takeshita K, Matsunaga S, Watanabe M, Nakagawa Y et al. Acute cervical spinal cord injury complicated by preexisting ossification of the posterior longitudinal ligament: a multicenter study. Spine 2011; 36: 1453–1458.
Katoh S, Ikata T, Hirai N, Okada Y, Nakauchi K . Influence of minor trauma to the neck on the neurological outcome in patients with ossification of the posterior longitudinal ligament (OPLL) of the cervical spine. Paraplegia 1995; 33: 330–333.
Matsunaga S, Sakou T, Hayashi K, Ishidou Y, Hirotsu M, Komiya S . Trauma-induced myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg 2002; 97: 172–175.
Trojan DA, Pouchot J, Pokrupa R, Ford RM, Adamsbaum C, Hill RO et al. Diagnosis and treatment of ossification of the posterior longitudinal ligament of the spine: report of eight cases and literature review. Am J Med 1992; 92: 296–306.
Matsunaga S, Sakou T . Ossification of the posterior longitudinal ligament of the cervical spine: etiology and natural history. Spine 2012; 37: E309–E314.
Matsunaga S, Sakou T, Taketomi E, Komiya S . Clinical course of patients with ossification of the posterior longitudinal ligament: a minimum 10-year cohort study. J Neurosurg 2004; 100: 245–248.
Terayama K, Kurokawa T, Seki H . National survey of ossification of the posterior longitudinal ligament. In: Investigation Committee 1975 Report on the Ossification of the Spinal Ligaments of the Japanese Ministry of Public Health and Welfare. Tokyo, Japan, 1976, pp 8–33.
Ueyama T, Tamaki N, Kondoh T, Miyamoto H, Akiyama H, Nagashima T . Non-traumatic acute paraplegia associated with cervical disc herniation: a case report. Surg Neurol 1999; 52: 204–206, discussion 206–207.
Suzuki T, Abe E, Murai H, Kobayashi T . Nontraumatic acute complete paraplegia resulting from cervical disc herniation: a case report. Spine 2003; 28: E125–E128.
Sadanand V, Kelly M, Varughese G, Fourney DR . Sudden quadriplegia after acute cervical disc herniation. Can J Neurol Sci 2005; 32: 356–358.
Liu C, Huang Y, Cai HX, Fan SW . Nontraumatic acute paraplegia associated with cervical disk herniation. J Spinal Cord Med 2010; 33: 420–424.
Cho JL, Park YS, Kim YH . Tetraparesis associated with ossification of the posterior longitudinal ligament of the cervical spine. Int Orthop 1999; 23: 247–248.
Cruzeiro MM, Vale TC, Pires LA, Franco GM, Pennisi MF . Tetraparesis secondary to cervical ossification of the posterior longitudinal ligament: case report. Arq Neuropsiquiatr 2007; 65: 532–535.
Rabinstein AA . Vascular myelopathies. Continuum 2015; 21: 67–83.
Rubin MN, Rabinstein AA . Vascular diseases of the spinal cord. Neurol Clin 2013; 31: 153–181.
Novy J, Carruzzo A, Maeder P, Bogousslavsky J . Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol 2006; 63: 1113–1120.
Cheshire WP, Santos CC, Massey EW, Howard JF Jr . Spinal cord infarction: etiology and outcome. Neurology 1996; 47: 321–330.
Rigney L, Cappelen-Smith C, Sebire D, Beran RG, Cordato D . Nontraumatic spinal cord ischaemic syndrome. J Clin Neurosci 2015; 22: 1544–1549.
Loher TJ, Bassetti CL, Lovblad KO, Stepper FP, Sturzenegger M, Kiefer C et al. Diffusion-weighted MRI in acute spinal cord ischaemia. Neuroradiology 2003; 45: 557–561.
Howard RS, Thorpe J, Barker R, Revesz T, Hirsch N, Miller D et al. Respiratory insufficiency due to high anterior cervical cord infarction. J Neurol Neurosurg Psychiatry 1998; 64: 358–361.
Mizuno J, Nakagawa H, Hashizume Y . Pathology of the spinal cord damaged by ossification of the posterior longitudinal ligament associated with spinal cord injury. Spinal Cord 1999; 37: 224–227.
Millichap JJ, Sy BT, Leacock RO . Spinal cord infarction with multiple etiologic factors. J Gen Intern Med 2007; 22: 151–154.
Westwick HJ, Goldstein CL, Shamji MF . Acute spontaneous cervical disc herniation causing rapidly progressive myelopathy in a patient with comorbid ossified posterior longitudinal ligament: case report and literature review. Surg Neurol Int 2014; 5: S368–S372.
Hsieh JH, Wu CT, Lee ST . Cervical intradural disc herniation after spinal manipulation therapy in a patient with ossification of posterior longitudinal ligament: a case report and review of the literature. Spine 2010; 35: E149–E151.
Cheong HS, Hong BY, Ko YA, Lim SH, Kim JS . Spinal cord injury incurred by neck massage. Ann Rehabil Med 2012; 36: 708–712.
Iwamura Y, Onari K, Kondo S, Inasaka R, Horii H . Cervical intradural disc herniation. Spine 2001; 26: 698–702.
Yoshizawa H . Presidential address: pathomechanism of myelopathy and radiculopathy from the viewpoint of blood flow and cerebrospinal fluid flow including a short historical review. Spine 2002; 27: 1255–1263.
Thurnher MM, Bammer R, Diffusion-weighted MR . imaging (DWI) in spinal cord ischemia. Neuroradiology 2006; 48: 795–801.
Reynolds JM, Belvadi YS, Kane AG, Poulopoulos M . Thoracic disc herniation leads to anterior spinal artery syndrome demonstrated by diffusion-weighted magnetic resonance imaging (DWI): a case report and literature review. Spine J 2014; 14: e17–e22.
Bhardwaj A, Long DM, Ducker TB, Toung TJ . Neurologic deficits after cervical laminectomy in the prone position. J Neurosurg Anesthesiol 2001; 13: 314–319.
Cybulski GR, D’Angelo CM . Neurological deterioration after laminectomy for spondylotic cervical myeloradiculopathy: the putative role of spinal cord ischaemia. J Neurol Neurosurg Psychiatry 1988; 51: 717–718.
Ben-David B, Haller G, Taylor P . Anterior spinal fusion complicated by paraplegia. A case report of a false-negative somatosensory-evoked potential. Spine 1987; 12: 536–539.
Kalb S, Fakhran S, Dean B, Ross J, Porter RW, Kakarla UK et al. Cervical spinal cord infarction after cervical spine decompressive surgery. World Neurosurg 2014; 81: 810–817.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tanida, A., Kamimura, A., Tanishima, S. et al. Spinal cord infarction at the level of ossification of the posterior longitudinal ligament. Spinal Cord Ser Cases 2, 16032 (2016). https://doi.org/10.1038/scsandc.2016.32
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/scsandc.2016.32